EXPLANATION
The presence of oxygen-containing compounds in gasoline can promote more complete combustion which reduces carbon monoxide emissions. The Clean Air Act of 1992 requires that gasoline sold within certain, specified geographical areas contains a minimum amount of oxygen (currently 2.7 mass %) during certain periods of the year. These test methods cover the determination of total oxygen in gasoline and methanol fuels. These test methods complement Test Method D4815, which covers the determination of several specific oxygen-containing compounds in gasoline. Several types of instruments can be used in these test methods. All pyrolyze the fuel in a carbon-rich environment. Instruments, however, differ in the way the oxygen-containing species are detected and quantitated.
TEST SUMMARY
A fuel sample is injected by syringe into a 950 to 1300° C high-temperature tube furnace that contains metallized carbon. Oxygen containing compounds are pyrolyzed, and the oxygen is quantitatively converted into carbon monoxide. A carrier gas such as N, He, or a He-H mixture, sweeps the pyrolysis gases into any of four downstream systems of reactors, scrubbers, separators, and detectors for the determination of the carbon monoxide content, and hence, of the oxygen in the original fuel sample.
TEST PRECISION
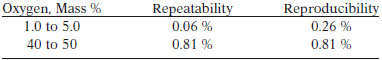
Based on the analysis of NIST SRM, this test method has no bias.

