GENERAL
The concentration of nitrogen is a measure of the presence of nitrogen-containing additives. Knowledge of its concentration can be used to predict performance.
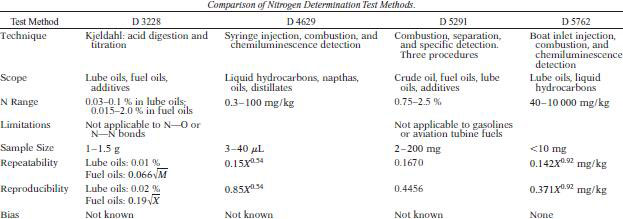
There are two ways of determining nitrogen in additives: Kjeldahl method and instrumental combustion method. A comparison of these methods is given in table below.
EXPLANATION
This test method covers the determination of the trace nitrogen naturally found in liquid hydrocarbons boiling in the range from approximately 50 to 400° C, with viscosities between approximately 0.2 and 10 cSt (mm2/s) at room temperature. This test method is applicable to naphthas, distillates, and oils containing 0.3 to 100 mg/kg total nitrogen.
TEST SUMMARY
The sample of liquid petroleum hydrocarbon is injected into a stream of inert gas (helium or argon). The sample is vaporized and carried to a high temperature zone where oxygen is introduced and organic and bound nitrogen is converted to nitric oxide (NO). The NO contacts ozone and is converted to excited nitrogen oxide (NO2). The light emitted as the excited NO2 decays is detected by a photomultiplier tube and the resulting signal is a measure of the nitrogen contained in the sample.
TEST PRECISION

Where X is the average of two test results.



