7.0 PROCEDURE
7.1 Sample extraction
7.1.1 Refer to Chapter Two and Method 3500 for guidance in choosing the appropriate extraction procedure. In general, water samples are extracted at a neutral pH with methylene chloride using a separatory funnel (Method 3510) or a continuous liquid-liquid extractor (Method 3520) or other appropriate procedure. Solid samples are extracted with hexane-acetone (1:1) or methylene chloride-acetone (1:1) using one of the Soxhlet extraction (Method 3540 or 3541) procedures, ultrasonic extraction (Method 3550), or other appropriate procedure.
NOTE:
Use of hexane-acetone generally reduces the amount of interferences that are extracted and improves signal-to-noise.
7.1.2 Reference materials, field-contaminated samples, or spiked samples should be used to verify the applicability of the selected extraction technique to each new sample type. Such samples should contain or be spiked with the compounds of interest in order to determine the percent recovery and the limit of detection for that sample type (see Chapter One). When other materials are not available and spiked samples are used, they should be spiked with the analytes of interest, either specific Aroclors or PCB congeners. When the presence of specific Aroclors is not anticipated, the Aroclor 1016/1260 mixture may be an appropriate choice for spiking. See Methods 3500 and 8000 for guidance on demonstration of initial method proficiency as well as guidance on matrix spikes for routine sample analysis.
7.2 Extract cleanup
Refer to Methods 3660 and 3665 for information on extract cleanup.
7.3 GC conditions
This method allows the analyst to choose between a single-column or a dual-column configuration in the injector port. Either wide- or narrow-bore columns may be used. See Sec.
7.7 for information on techniques for making positive identifications of multi-component analytes.
7.3.1 Single-column analysis
This capillary GC/ECD method allows the analyst the option of using 0.25-0.32 mm ID capillary columns (narrow-bore) or 0.53 mm ID capillary columns (wide-bore). The use of narrow-bore (0.25-0.32 mm ID) columns is recommended when the analyst requires greater chromatographic resolution. Use of narrow-bore columns is suitable for relatively clean samples or for extracts that have been prepared with one or more of the clean-up options referenced in the method. Wide-bore columns (0.53 mm ID) are suitable for more complex environmental and waste matrices.
7.3.2 Dual-column analysis
The dual-column/dual-detector approach involves the use of two 30 m x 0.53 mm ID fused-silica open-tubular columns of different polarities, thus different selectivities towards the target compounds. The columns are connected to an injection tee and ECD detectors.
7.3.3 GC temperature programs and flow rates
7.3.3.1 Table 2 lists GC operating conditions for the analysis of PCBs as Aroclors for single-column analysis, using either narrow-bore or wide-bore capillary columns. Table 3 lists GC operating conditions for the dual-column analysis. Use the conditions in these tables as guidance and establish the GC temperature program and flow rate necessary to separate the analytes of interest.
7.3.3.2 When determining PCBs as congeners, difficulties may be encountered with coelution of congener 153 and other sample components. When determining PCBs as Aroclors, chromatographic conditions should be adjusted to give adequate separation of the characteristic peaks in each Aroclor (see Sec. 7.4.6).
7.3.3.3 Tables 4 and 5 summarize the retention times of up to 73 Aroclor peaks determined during dual-column analysis using the operating conditions listed in Table 2. These retention times are provided as guidance as to what may be achieved using the GC columns, temperature programs, and flow rates described in this method. Note that the peak numbers used in these tables are not the IUPAC congener numbers, but represent the elution order of the peaks on these GC columns.
7.3.3.4 Once established, the same operating conditions must be used for the analysis of samples and standards.
7.4 Calibration
7.4.1 Prepare calibration standards as described in Sec. 5.0. Refer to Method 8000 (Sec. 7.0) for proper calibration techniques for both initial calibration and calibration verification. When PCBs are to be determined as congeners, the use of internal standard calibration is highly recommended. Therefore, the calibration standards must contain the internal standard (see Sec. 5.7) at the same concentration as the sample extracts. When PCBs are to be determined as Aroclors, external standard calibration should be used.
NOTE:
Because of the sensitivity of the electron capture detector, the injection port and column should always be cleaned prior to performing the initial calibration.
7.4.2 When PCBs are to be quantitatively determined as congeners, an initial five-point calibration must be performed that includes standards for all the target analytes (congeners).
7.4.3 When PCBs are to be quantitatively determined as Aroclors, the initial calibration consists of two parts, described below.
7.4.3.1 As noted in Sec. 5.6.1, a standard containing a mixture of Aroclor 1016 and Aroclor 1260 will include many of the peaks represented in the other five Aroclor mixtures. Thus, such a standard may be used to demonstrate the linearity of the detector and that a sample does not contain peaks that represent any one of the Aroclors. This standard can also be used to determine the concentrations of either Aroclor 1016 or Aroclor 1260, should they be present in a sample. Therefore, an initial five-point calibration is performed using the mixture of Aroclors 1016 and 1260 described in Sec. 5.6.1.
7.4.3.2 Standards of the other five Aroclors are necessary for pattern recognition. These standards are also used to determine a single-point calibration factor for each Aroclor, assuming that the Aroclor 1016/1260 mixture in Sec. 7.3.4.1 has been used to describe the detector response. The standards for these five Aroclors should be analyzed before the analysis of any samples, and may be analyzed before or after the analysis of the five 1016/1260 standards in Sec. 7.3.4.1.
7.4.3.3 In situations where only a few Aroclors are of interest for a specific project, the analyst may employ a five-point initial calibration of each of the Aroclors of interest (e.g., five standards of Aroclor 1232 if this Aroclor is of concern) and not use the 1016/1260 mixture described in Sec. 7.4.3.1 or the pattern recognition standards described in 7.4.3.2.
7.4.4 Establish the GC operating conditions appropriate for the configuration (single-column or dual column, Sec. 7.3). Optimize the instrumental conditions for resolution of the target compounds and sensitivity. A final temperature of 240-270EC may be required to elute decachlorobiphenyl. Use of injector pressure programming will improve the chromatography of late eluting peaks.
NOTE:
Once established, the same operating conditions must be used for both calibrations and sample analyses.
7.4.5 A 2-µL injection of each calibration standard is recommended. Other injection volumes may be employed, provided that the analyst can demonstrate adequate sensitivity for the compounds of interest.
7.4.6 Record the peak area (or height) for each congener or each characteristic Aroclor peak to be used for quantitation.
7.4.6.1 A minimum of 3 peaks must be chosen for each Aroclor, and preferably 5 peaks. The peaks must be characteristic of the Aroclor in question. Choose peaks in the Aroclor standards that are at least 25% of the height of the largest Aroclor peak. For each Aroclor, the set of 3 to 5 peaks should include at least one peak that is unique to that Aroclor. Use at least five peaks for the Aroclor 1016/1260 mixture, none of which should be found in both of these Aroclors.
7.4.6.2 Late-eluting Aroclor peaks are generally the most stable in the environment. Table 6 lists diagnostic peaks in each Aroclor, along with their retention times on two GC columns suitable for single-column analysis. Table 7 lists 13 specific PCB congeners found in Aroclor mixtures. Table 8 lists PCB congeners with corresponding retention times on a DB-5 wide-bore GC column. Use these tables as guidance in choosing the appropriate peaks.
7.4.7 When determining PCB congeners by the internal standard procedure, calculate he response factor (RF) for each congener in the calibration standards relative to the internal standard, decachlorobiphenyl, using the equation that follows.
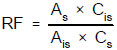
where:
As = Peak area (or height) of the analyte or surrogate.
Ais = Peak area (or height) of the internal standard.
Cs = Concentration of the analyte or surrogate, in µg/L.
Cis = Concentration of the internal standard, in µg/L.
7.4.8 When determining PCBs as Aroclors by the external standard technique, calculate the calibration factor (CF) for each characteristic Aroclor peak in each of the initial calibration standards (from either Sec. 7.4.3.1 or 7.4.3.2) using the equation below.

Five sets of calibration factors will be generated for the Aroclor 1016/1260 mixture, each set consisting of the calibration factors for each of the five (or more) peaks chosen for this mixture. The single standard for each of the other Aroclors (see Sec. 7.4.3.1) will generate at least three calibration factors, one for each selected peak.
7.4.9 The response factors or calibration factors from the initial calibration are used to evaluate the linearity of the initial calibration. This involves the calculation of the mean response or calibration factor, the standard deviation, and the relative standard deviation (RSD) for each congener or Aroclor peak. See Method 8000 for the specifics of the evaluation of the linearity of the calibration and guidance on performing non-linear calibrations. When the Aroclor 1016/1260 mixture is used to demonstrate the detector response, the calibration model (see Method 8000) chosen for this mixture must be applied to the other five Aroclors for which only single standards are analyzed. If multi-point calibration is performed for individual Aroclors (see Sec. 7.4.3.3), use the calibration factors from those standards to evaluate linearity.
7.5 Retention time windows
Retention time windows are crucial to the identification of target compounds. Absolute retention times are used for the identification of PCBs as Aroclors. When PCBs are determined as congeners by an internal standard technique, absolute retention times may be used in conjunction with relative retention times (relative to the internal standard). Retention time windows are established to compensate for minor shifts in absolute retention times as a result of sample loadings and normal chromatographic variability. The width of the retention time window should be carefully established to minimize the occurrence of both false positive and false negative results. Tight retention time windows may result in false negatives and/or may cause unnecessary reanalysis of samples when surrogates or spiked compounds are erroneously not identified. Overly wide retention time windows may result in false positive results that cannot be confirmed upon further analysis. Analysts should consult Method 8000 for the details of establishing retention time windows.
7.6 Gas chromatographic analysis of sample extracts
7.6.1 The same GC operating conditions used for the initial calibration must be employed for samples analyses.
7.6.2 Verify calibration each 12-hour shift by injecting calibration verification standards prior to conducting any sample analyses. A calibration standard must also be injected at intervals of not less than once every twenty samples (after every 10 samples is recommended to minimize the number of samples requiring re-injection when QC limits are exceeded) and at the end of the analysis sequence. For Aroclor analyses, the calibration verification standard should be a mixture of Aroclor 1016 and Aroclor 1260. The calibration verification process does not require analysis of the other Aroclor standards used for pattern recognition, but the analyst may wish to include a standard for one of these Aroclors after the 1016/1260 mixture used for calibration verification throughout the analytical sequence.
7.6.2.1 The calibration factor for each analyte calculated from the calibration verification standard (CFv) must not exceed a difference of more than ± 15 percent when compared to the mean calibration factor from the initial calibration curve.
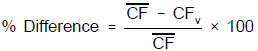
7.6.2.2 When internal standard calibration is used for PCB congeners, the response factor calculated from the calibration verification standard (RFv) must not exceed a ± 15 percent difference when compared to the mean response factor from the initial calibration

7.6.2.3 If this criterion is exceeded for any calibration factor or response factor, inspect the gas chromatographic system to determine the cause and perform whatever maintenance is necessary before verifying calibration and proceeding with sample analysis.
7.6.2.4 If routine maintenance does not return the instrument performance to meet the QC requirements (Sec. 8.2) based on the last initial calibration, then a new initial calibration must be performed.
7.6.3 Inject a 2-µL aliquot of the concentrated sample extract. Record the volume injected to the nearest 0.05 µL and the resulting peak size in area (or peak height) units.
7.6.4 Qualitative identifications of target analytes are made by examination of the sample chromatograms, as described in Sec. 7.7.
7.6.5 Quantitative results are determined for each identified analyte (Aroclors or congeners), using the procedures described in Secs. 7.8 and 7.9 for either the internal or the external calibration procedure (Method 8000). If the responses in the sample chromatogram exceed the calibration range of the system, dilute the extract and reanalyze. Peak height measurements are recommended over peak area when overlapping peaks cause errors in area integration.
7.6.6 Each sample analysis must be bracketed with an acceptable initial calibration, calibration verification standard(s) (each 12-hour shift), or calibration standards interspersed within the samples. When a calibration verification standard fails to meet the QC criteria, all samples that were injected after the last standard that last met the QC criteria must be re-injected.
Multi-level standards (mixtures or multi-component analytes) are highly recommended to ensure that detector response remains stable for all analytes over the calibration range.
7.6.7 Sample injections may continue for as long as the calibration verification standards and standards interspersed with the samples meet instrument QC requirements. It is recommended that standards be analyzed after every 10 samples (required after every 20 samples and at the end of a set) to minimize the number of samples that must be re-injected when the standards fail the QC limits. The sequence ends when the set of samples has been injected or when qualitative or quantitative QC criteria are exceeded.
7.6.8 If the peak response is less than 2.5 times the baseline noise level, the validity of the quantitative result may be questionable. The analyst should consult with the source of the sample to determine whether further concentration of the sample is warranted.
7.6.9 Use the calibration standards analyzed during the sequence to evaluate retention time stability. If any of the standards fall outside their daily retention time windows, the system is out of control. Determine the cause of the problem and correct it.
7.6.10 If compound identification or quantitation is precluded due to interference (e.g., broad, rounded peaks or ill-defined baselines are present) cleanup of the extract or replacement of the capillary column or detector is warranted. Rerun the sample on another instrument to determine if the problem results from analytical hardware or the sample matrix. Refer to Method 3600 for the procedures to be followed in sample cleanup.
7.7 Qualitative identification
The identification of PCBs as either Aroclors or congeners using this method with an electron capture detector is based on agreement between the retention times of peaks in the sample chromatogram with the retention time windows established through the analysis of standards of the target analytes. See Method 8000 for information on the establishment of retention time windows.
Tentative identification of an analyte occurs when a peak from a sample extract falls within the established retention time window for a specific target analyte. Each tentative identification must be confirmed: using a second GC column of dissimilar stationary phase (as in the dual-column analysis), based on a clearly identifiable Aroclor pattern, or using another technique such as GC/MS (see Sec. 7.10).
7.7.1 When simultaneous analyses are performed from a single injection (the dual-column GC configuration described in Sec. 7.3), it is not practical to designate one column as the analytical (primary) column and the other as the confirmation column. Since the calibration standards are analyzed on both columns, the results for both columns must meet the calibration acceptance criteria. If the retention times of the peaks on both columns fall within the retention time windows on the respective columns, then the target analyte identification has been confirmed.
7.7.2 The results of a single column/single injection analysis may be confirmed on a second, dissimilar, GC column. In order to be used for confirmation, retention time windows must have been established for the second GC column. In addition, the analyst must demonstrate the sensitivity of the second column analysis. This demonstration must include the analysis of a standard of the target analyte at a concentration at least as low as the concentration estimated from the primary analysis. That standard may be either the individual congeners, individual Aroclor or the Aroclor 1016/1260 mixture.
7.7.3 When samples are analyzed from a source known to contain specific Aroclors, the results from a single-column analysis may be confirmed on the basis of a clearly recognizable Aroclor pattern. This approach should not be attempted for samples from unknown or unfamiliar sources or for samples that appear to contain mixtures of Aroclors. In order to employ this approach, the analyst must document:
• The peaks that were evaluated when comparing the sample chromatogram and the Aroclor standard.
• The absence of major peaks representing any other Aroclor.
• The source-specific information indicating that Aroclors are anticipated in the sample (e.g., historical data, generator knowledge, etc.).
This information should either be provided to the data user or maintained by the laboratory.
7.7.4 See Sec. 7.10 for information on GC/MS confirmation.
7.8 Quantitation of PCBs as congeners
7.8.1 The quantitation of PCB congeners is accomplished by the comparison of the sample chromatogram to those of the PCB congener standards, using the internal standard technique (see Method 8000). Calculate the concentration of each congener.
7.8.2 Depending on project requirements, the PCB congener results may be reported as congeners, or may be summed and reported as total PCBs. The analyst should use caution when using the congener method for quantitation when regulatory requirements are based on Aroclor concentrations. See Sec. 9.3.
7.9 Quantitation of PCBs as Aroclors
The quantitation of PCB residues as Aroclors is accomplished by comparison of the sample chromatogram to that of the most similar Aroclor standard. A choice must be made as to which Aroclor is most similar to that of the residue and whether that standard is truly representative of the PCBs in the sample.
7.9.1 Use the individual Aroclor standards (not the 1016/1260 mixtures) to determine the pattern of peaks on Aroclors 1221, 1232, 1242, 1248, and 1254. The patterns for Aroclors 1016 and 1260 will be evident in the mixed calibration standards.
7.9.2 Once the Aroclor pattern has been identified, compare the responses of 3 to 5 major peaks in the single-point calibration standard for that Aroclor with the peaks observed in the sample extract. The amount of Aroclor is calculated using the individual calibration factor for each of the 3 to 5 characteristic peaks chosen in Sec. 7.4.6.1. and the calibration model (linear or non-linear) established from the multi-point calibration of the 1016/1260 mixture. A concentration is determined using each of the characteristic peaks and then those 3 to 5 concentrations are averaged to determine the concentration of that Aroclor.
7.9.3 Weathering of PCBs in the environment and changes resulting from waste treatment processes may alter the PCBs to the point that the pattern of a specific Aroclor is no longer recognizable. Samples containing more than one Aroclor present similar problems. If the purpose of the analysis is not regulatory compliance monitoring on the basis of Aroclor concentrations, then it may be more appropriate to perform the analyses using the PCB congener approach described in this method. If results in terms of Aroclors are required, then the quantitation as Aroclors may be performed by measuring the total area of the PCB pattern and quantitating on the basis of the Aroclor standard that is most similar to the sample. Any peaks that are not identifiable as PCBs on the basis of retention times should be subtracted from the total area. When quantitation is performed in this manner, the problems should be fully described for the data user and the specific procedures employed by the analyst should be thoroughly documented.
7.10 GC/MS confirmation may be used in conjunction with either single-or dual-column analysis if the concentration is sufficient for detection by GC/MS.
7.10.1 Full-scan quadrupole GC/MS will normally require a higher concentration of the analyte of interest than full-scan ion trap or selected ion monitoring techniques. The concentrations will be instrument-dependent, but values for full-scan quadrupole GC/MS may be as high as 10 ng/µL in the final extract, while ion trap or SIM may only require a concentration of 1 ng/µL.
7.10.2 The GC/MS must be calibrated for the specific target analytes. When using SIM techniques, the ions and retention times should be characteristic of the Aroclors to be confirmed.
7.10.3 GC/MS confirmation should be accomplished by analyzing the same extract used for GC/ECD analysis and the extract of the associated blank.
7.10.4 The base/neutral/acid extract and the associated blank may be used for GC/MS confirmation if the surrogates and internal standards do not interfere. However, if the compounds are not detected in the base/neutral/acid extract, then GC/MS analysis of the pesticide extract should be performed.
7.10.5 A QC reference sample containing the compound must also be analyzed by GC/MS. The concentration of the QC reference sample must demonstrate that those PCBs identified by GC/ECD can be confirmed by GC/MS.
7.11 Chromatographic System Maintenance as Corrective Action
When system performance does not meet the established QC requirements, corrective action is required, and may include one or more of the following.
7.11.1 Splitter connections
For dual columns which are connected using a press-fit Y-shaped glass splitter or a Y-shaped fused-silica connector, clean and deactivate the splitter port insert or replace with a cleaned and deactivated splitter. Break off the first few inches (up to one foot) of the injection port side of the column. Remove the columns and solvent backflush according to the manufacturer's instructions. If these procedures fail to eliminate the degradation problem, it may be necessary to deactivate the metal injector body and/or replace the columns.
7.11.2 Metal injector body
Turn off the oven and remove the analytical columns when the oven has cooled. Remove the glass injection port insert (instruments with on-column injection). Lower the injection port temperature to room temperature. Inspect the injection port and remove any noticeable foreign material.
7.11.2.1 Place a beaker beneath the injector port inside the oven. Using a wash bottle, rinse the entire inside of the injector port with acetone and then rinse it with toluene, catching the rinsate in the beaker.
7.11.2.2 Consult the manufacturer's instructions regarding deactivating the injector port body. Glass injection port liners may require deactivation with a silanizing solution containing dimethyldichlorosilane.
7.11.3 Column rinsing
The column should be rinsed with several column volumes of an appropriate solvent. Both polar and nonpolar solvents are recommended. Depending on the nature of the sample residues expected, the first rinse might be water, followed by methanol and acetone. Methylene chloride is a good final rinse and in some cases may be the only solvent required. The column should then be filled with methylene chloride and allowed to stand flooded overnight to allow materials within the stationary phase to migrate into the solvent. The column is then flushed with fresh methylene chloride, drained, and dried at room temperature with a stream of ultrapure nitrogen.

