1 Scope
This part of IEC 62697 specifies a test method for the quantitative determination of corrosive sulfur compounds-dibenzyl disulfide (DBDS) in used and unused insulating liquids over a 5 - 600 mg kg(-1) concentration range.
2 Normative references
The following documents, in whole or in part, are normatively referenced in this document and are indispensable for its application. For dated references, only the edition cited applies. For undated references, the latest edition of the referenced document (including any amendments) applies.
IEC 60475, Method of sampling liquid dielectrics
IEC 62535:2008, Insulating liquids - Test method for detection of potentially corrosive sulfur in used and unused insulating oil
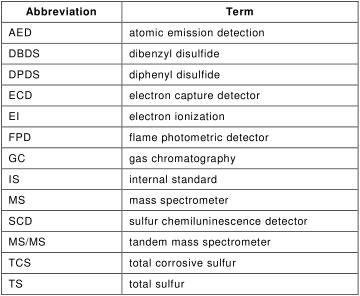
3 Terms, definitions and abbreviations
For the purposes of this document, the following terms, definitions and abbreviations apply.
3.1 Terms and definitions
3.1.1
accuracy
closeness of agreement between test result and the accepted reference value
3.1.2
additive
a suitable chemical substance that is deliberately added to insulating liquid in order to improve certain characteristics
Note 1 to entry: Examples include antioxidants, pour-point depressants, electrostatic charging tendency depressant such as benzotriazol (BTA) metal passivator or deactivators, antifoam agent, refining process improver, etc.
3.1.3
atomic emission detector
AED
simultaneously monitors emissions of radiation resulting from atomic species excited in a microwave-induced plasma and permits quantitative determination of selected heteroatoms in compounds that elute from a GC column
Note 1 to entry: AED thus provides heteroatom profiles, i.e. "fingerprints" of complex samples such as insulating liquids.
3.1.4
contaminants
foreign substances or materials in an insulating liquid or gas which usually has a deleterious effect on one or more properties
[SOURCE: IEC 60050-212:2010, 212-17-27, modified]
3.1.5
corrosion
disintegration of a metal due to chemical reactions with sulfur and other chemical species in insulating liquids
3.1.6
corrosive sulfur
free sulfur and corrosive sulfur compounds detected by subjecting metals such as copper to contact with an insulating liquid under standardized conditions
[SOURCE: IEC 60050-212:2010, 212-18-20]
3.1.7
dibenzyl disulfide
DBDS
aromatic disulfide containing two benzyl functionalities with a molecular formula C14H14S2, nominal molecular mass of 246 and a melting point of 71 - 72 °C
3.1.8
diphenyl disulfide
DPDS
aromatic disulfide with two phenyl functionalities with a molecular formula C12H10S2, nominal molecular mass of 218 and a melting point of 61 °C - 62 ° C
3.1.9
electron capture detector
ECD
device used for quantification of compounds with high electron affinity such as polychlorinated aromatics, nitroaromatics and aromatic disulfides present in gas chromatography effluent at very low concentrations
Note 1 to entry: ECD can have a radioactive internal ionization source (e.g. 63Ni) or thermal electron produced through photo-induced ionization (e.g. helium discharge - HD or photoionization - PID).
3.1.10
flame photometric detector
FPD
detector that uses the chemiluminescent reaction of sulfur-containing compounds in a cool hydrogen/air flame that result in the formation of excited S2* species, which decays with broad radiant out around 394 nm that is monitored with an interference filter and a photomultiplier
3.1.11
homologue
compound belonging to a series of compounds that differ in the number of repeating groups
3.1.12
internal standard
IS
substance which is similar in the chemical behaviour (chemical structure - polarity) and analytical response to a certain target analyte
Note 1 to entry: A defined volume of the internal standard solution is added to both the sample and calibration solutions such that they both contain an identical concentration.
3.1.13
isomer
compounds that have the same molecular formula but different structural formula
3.1.14
gas chromatograph
device used for separating volatile and semi-volatile compounds in mixtures that can be vaporized without decomposition through differential migration with a carrier gas through a column
3.1.15
mass spectrometer
MS
instrument used for ionizing neutral chemical species and separating ions according to their mass to charge ratio
Note 1 to entry: It permits determining concentrations of target compounds in complex mixtures such as insulating liquids.
3.1.16
mercaptans (thiols) and disulfides
corrosive organic compounds that contain the functional group composed of a sulfur-hydrogen bond (-SH); disulfides are corrosive compounds that contain a linked pair of sulfur atoms (S-S, disulfide bond)
3.1.17
precision
closeness of agreement between independent test results obtained under stipulated conditions (repeatability conditions or reproducibility conditions)
3.1.18
potentially corrosive sulfur
organo-sulfur compounds present in transformer oils that may cause copper sulfide formation
Note 1 to entry: Some of these compounds may be initially corrosive, or become corrosive under certain operating conditions.
[SOURCE: IEC 62535:2008, 3.1]
3.1.19
qualitative analysis
analysis that establishes the presence or the absence of a compound in a sample
3.1.20
quantitative analysis
analysis that establishes the amount or concentration of a compound in a sample
3.1.21
repeatability conditions
conditions where independent test results are obtained with the same method on identical test items in the same laboratory
3.1.22
repeatability limits
r
value less than or equal to which the absolute difference between two test results obtained under repeatability conditions may be expected to be with a probability of 95 %
3.1.23
reproducibility conditions
conditions where independent test results are obtained with the same method on identical test items in different laboratories with different operators using different equipment
3.1.24
reproducibility limits
R
value less than or equal to which the absolute difference between two test results obtained under reproducible conditions may be expected to be with a probability of 95 %
3.1.25
sulfur chemiluminescence detector
SCD
detector that makes use of a dual plasma burner to combust sulfur-containing compounds to yield sulfur monoxide (SO)
Note 1 to entry: A photomultiplier tube detects the light produced by the chemiluminescent reaction of SO with ozone. This results in a linear and equimolar response to the sulfur compounds without interference from most sample matrices.
3.1.26
tandem mass spectrometer
MS/MS
system that permits selection of specific precursor ion/s and dissociation of these ions to produce characteristic fragment ion/s
Note 1 to entry: Monitoring of fragment ions permits matrix interference-free quantification of targeted compounds in complex samples.
3.1.27
total corrosive sulfur
TCS
sum of all free and chemically bound sulfur in an insulating liquid that reacts with metals such as copper under certain operating conditions
3.1.28
total sulfur
TS
sum of all free sulfur and chemically bound sulfur present in an insulating liquid
3.1.29
trueness
closeness of agreement between the average value obtained from large series of test results and an accepted reference value
3.1.30
unused mineral insulating oil
mineral insulating oil as delivered by the supplier
Note 1 to entry: Such oil should not have been used in, nor been in contact with, electrical equipment not required for manufacture, storage or transportation.
Note 2 to entry: The manufacturer and supplier of unused oil will have taken all reasonable precautions to ensure that there is no contamination with polychlorinated biphenyls or terphenyls (PCBs, PCTs), used, reclaimed or dechlorinated oil or other contaminants
[SOURCE: IEC 60296:2012, definition 3.9, modified]
3.2 Abbreviations