8 Procedure
Rinse and fill the burette with 0,05 mol/l alcoholic potassium hydroxide solution (5.1).
Standardize the alcoholic potassium hydroxide solution at least every two weeks against potassium hydrogen phthalate (5.3) or certified standard 0,1 mol/l acid.
Carry out a blank titration on the solvent (5.2) each day and after changing to a fresh batch of solvent.
8.1 Standardization of alcoholic potassium hydroxide solution
Standardize the alcoholic potassium hydroxide solution, using a suitable indicator, against 0,1 g to 0,16 g of potassium hydrogen phthalate, weighed to an accuracy of 0,0002 g and dissolved in approximately 100 ml of carbon dioxide free water.
Alternatively the standardization can be performed by potentiometric titration.
Calculate the molarity M to the nearest 0,0005 using Equation (1):
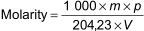
where
m is the mass of potassium hydrogen phthalate, in grams;
p is the percent purity of the potassium hydrogen phthalate;
V is the volume of potassium hydroxide solution, in millilitres.
Alternatively, certified standard 0,1 mol/l acid may be used to standardize the alcoholic potassium hydroxide solution.
Calculate the molarity M to the nearest 0,0005 using Equation (2):
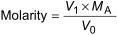
where
V1 is the volume of 0,1 mol/l standard acid used to titrate the solution, in millilitres;
MA is the molarity of the standard hydrochloric acid;
V0 is the volume of potassium hydroxide solution, in millilitres.
8.2 Blank titration
Perform a blank titration at a temperature not above 25 °C on 10 ml +/- 0,1 ml aliquots of the solvent containing 0,5 % of alkali blue 6B indicator solution (5.5) using the standardized alcoholic potassium hydroxide solution. The endpoint shall be as soon as a colour change from blue to a red colour comparable to that of the cobalt nitrate solution (5.6) is obtained and persists for at least 15 s.
Carry out triplicate titrations and calculate the mean result, in millilitres to the nearest 0,001 ml, as the blank value V0.
Protect the solvent from atmospheric carbon dioxide and use within 8 h.
8.3 Sample titration
Weigh 5 g of sample to the nearest 0,01 g into the titration vessel. Add 10 ml +/- 0,1 ml of the solvent solution containing 0,5 % of alkali blue 6B indicator solution (5.5). Swirl to dissolve the oil and immediately titrate at a temperature not above 25 °C with the standardized potassium hydroxide solution. A typical end point is as described in 8.2. However, since the colour change may vary for different oils, pre-titration may be necessary to establish this. In such cases, the endpoint shall be reached as soon as a stable colour change, which persists for at least 15 s, is obtained.
NOTE Before titrating, the colour may vary from blue to green and at the endpoint from red to light orange to dark yellow-brown, depending on the original colour of the oil.
Carry out determinations for each oil sample and note the result, in millilitres, to the nearest 0,001 ml, as the titration value V1.

