10 Procedure
Set up the apparatus in accordance with the manufacturer's instructions.
Rinse and fill the burette with 0,05 mol/l (or 0,1 mol/l as in 5.2) alcoholic potassium hydroxide solution (see 5.2).
Standardize the 0,05 mol/l alcoholic potassium hydroxide solution regularly against potassium hydrogen phthalate (see 10.1).
10.1 Standardization of alcoholic potassium hydroxide solution
Standardize the alcoholic potassium hydroxide solution potentiometrically against 0,1 g to 0,16 g of the potassium hydrogen phthalate, weighed to an accuracy of 0,0002 g and dissolved in approximately 100 ml of carbon dioxide free water.
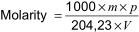
where
m is the mass of potassium hydrogen phthalate, in g;
p is the purity of potassium hydrogen phthalate;
204,23 is the molecular weight of potassium hydrogen phthalate;
V is the volume of alcoholic KOH solution (see 5.2) used to titrate the solution, in ml.
Alternatively, standard 0,1 mol/l acid may be used to standardize the alcoholic KOH (see note in 5.4).
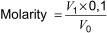
where
V1 is the volume of 0,1 mol/l standard acid used to titrate the solution, in ml;
V0 is the volume of potassium hydroxide solution, in ml.
10.2 Blank titration
Perform a blank titration in duplicate as in 10.3, on 20 ml +/- 0,1 ml of the solvent (see 5.3) daily and after changing to a fresh batch of solvent.
Blank titrations shall be continued until two consecutive titrations differ by no more than 0,005 ml, based on 20 ml of solvent and the mean of these is calculated as V0 (see Clause 11).
Where a higher solvent volume than 20 ml is required because of apparatus constraints, the same volume of solvent shall be used for the sample titration.
High values may arise from carbon dioxide absorption or inherent 2-propanol acidity. If the blank value is greater than 0,06 ml (based on 20 ml of solvent), steps shall be taken to remove the cause of the high values.
10.3 Sample titration
Prepare the sample for titration as described in Clause 7 and weigh 5 g +/- 0,1 g of the mineral oil to the nearest 0,01 g into the titration vessel. Add 20 ml +/- 0,1 ml of titration solvent (see 5.3).
Place the titration vessel on the titration stand and stir the solution until the sample has dissolved and the pH reading is constant, taking care to limit the speed of stirring to avoid spattering and/or stirring air into the solution.
Carry out the titration with 0,05 mol/l (or 0,1 mol/l as in 5.2) potassium hydroxide, following the instrument manufacturer's recommendations, to an end-point of pH 11,5 or higher if the instrument cannot be set for a dead-stop titration.
NOTE 1 Dynamic titrant addition is preferred to reduce the overall analysis time.
NOTE 2 When the titration time exceeds 15 min, it may be necessary to prevent carbon dioxide absorption by blanketing the solution with nitrogen.
On completion of the titration, record the burette reading V1 (see Clause 11) at the pH readingof 11,5.
Rinse the electrodes and burette tip with titration solvent (see 5.3). Re-hydrate the glass electrode by immersing the bulb in de-ionized water (see 8.1) and allow excess water to drain off. Where oxidized oil is analysed, the electrode should be immersed in de-ionized water containing a few drops of hydrochloric acid, followed by rinsing in de-ionized water.
If further titrations are not to be carried out immediately, the electrodes shall be stored in the de-ionized water.

