5 Reagents and auxiliary materials
5.1 Reagents and standards
All reagents and materials, including those for clean-up, shall be free from PCB contamination and compounds responding to the ECD.
5.1.1 Solvent
Hexane, heptane, cyclohexane or isooctane (2,2,4-trimethylpentane), high purity, free from PCB contamination and low in compounds that respond to the ECD.
5.1.2 Hexachlorobenzene
Purity 99 % at least, used for checking detector sensitivity.
5.1.3 Insulating liquid
An insulating liquid, checked to be free from PCBs or other interfering substances, of the same type as is present in the sample.
5.1.4 Congener 30 solution (C 30)
10 mg/l in solvent (5.1.1) purchased in solution or prepared from pure material (purity 99 % at least).
5.1.5 Congener 209 (DCB), decachlorobiphenyl solution
10 mg/l in solvent (5.1.1) purchased in solution or prepared from pure material (purity 99 % at least).
5.1.6 Calibration solution of selected PCB congeners
Certified calibration mixture in solvent (5.1.1) containing at least the following PCB congeners at a concentration of 10 mg/l each: 18, 28, 31, 44, 52, 101, 118, 138, 149, 153, 170, 180, 194 and 209 (see B.3).
5.2 Commercial PCB standards (see B.4)
5.2.1 Solutions of Aroclors ® 1242, 1254 and 1260 in solvent (5.1.1)
Concentration required 50 mg/l or more, typically 1 000 mg/l.
5.2.2 Solutions of Aroclors ® 1242, 1254 and 1260 in oil
50 mg/kg solutions of Aroclors ® 1242, 1254 and 1260 in unused insulating liquid, either purchased as standardized solutions or prepared from pure material.
5.3 Gas chromatography gases
5.3.1 Carrier gas: helium or hydrogen, purity 99,99 % at least.
5.3.2 Make-up gas: argon/methane, 95 %/5 %. Alternatively, 99,99 % minimum purity nitrogen can be used.
5.4 Internal standard/reference solutions
NOTE Standards should be stored in a cool, dark place.
5.4.1 Internal standard solution 2 (IS 2)
2 mg/l C209 (DCB), 2 mg/l C30.
Pipette (5.8.3) 5 ml of DCB solution (5.1.5) and 5 ml C30 solution (5.1.4) into a 25 ml volumetric flask, make up to the mark with solvent (5.1.1).
5.4.2 Internal standard solution 0,5 (IS 0,5)
0,5 mg/l C209 (DCB), 0,5 mg/l C30.
Follow 5.4.1 using a 100 ml volumetric flask.
5.5 Test mixture solution (for system evaluation)
Into a 20 ml volumetric flask: weigh, to the nearest 0,001 g, 0,50 g of 50 mg/kg Aroclor ® 1260, plus 0,50 g of 50 mg/kg Aroclor ® 1254 plus 1,00 g of 50 mg/kg Aroclor ® 1242 solutions in insulating liquid (5.2.2).
Add by pipette 1 ml of IS 2 solution (5.4.1) and make up to volume with solvent.
Prior to use this solution shall be treated as per 11.1.3.
5.6 Calibration - congener mix stock solution
Into a 20 ml volumetric flask: weigh, to the nearest 0,001 g, 2,0 g of insulating liquid (5.1.3) and add 1 ml of the calibration PCB congener mix (5.1.6). Make up to the mark with solvent (5.1.1).
5.7 Congener mix calibration solution (for response factors)
Submit 500 μl of solution (5.6) to the clean-up (11.1.3). The final solution is suitable for the determination of relative factors.
Prepare a fresh solution monthly.
5.8 Glassware
5.8.1 Volumetric flasks 100, 50, 25, 10 and 5 ml (tolerance better than +/- 0,4 %)
5.8.2 Syringes and pipettes:
500 μl +/- 5 μl syringe or pipette,
1 μl and 5 μl gas chromatography precision syringes.
5.8.3 Bulb pipettes (volumetric) 1, 2 and 5 ml class A
5.9 Columns and accessories for sample preparation
5.9.1 Commercial or self-packed solid-phase extraction columns:
3 ml silica gel column, adsorbent weight 500 mg, particle size 40 μm,
3 ml benzenesulphonic acid column, adsorbent weight 500 mg, particle size 40 μm.
5.9.2 Column adapter, for joining two columns
5.9.3 Vacuum manifold column processor - optional
6 Apparatus
6.1 Gas chromatograph (GC)
A high-resolution gas chromatograph with accurately reproducible oven temperature control, capable, when used with the appropriate column and conditions, of resolving the test mixture (5.5) at least as well as in Figure A.1 (90 peaks observed) and of reproducing relative retention times to within +/- 0,0015.
The gas lines (carrier gas and make-up gas) shall be fitted with water vapour and oxygen traps.
The carrier gas supply system shall be capable of running with a 50 m column at maximum efficiency using He or H2 carrier gas, e.g. adequate column head pressure.
The oven temperature programmer shall have a range that can be set to attain the required resolution.
6.1.1 Injector
Either an "on-column" injector or a "split/splitless" injector may be used.
6.1.2 Columns
Either a cross-linked 5 % phenyl-methyl silicone, stationary phase coated onto fused silica capillary column or a similar chemically bonded phase column. Their dimensions shall be as follows:
length = 50 m to 60 m;
internal diameter = 0,2 mm to 0,35 mm;
film thickness = 0,1 μm to 0,25 μm.
NOTE For suitable columns and manufacturers see B.2.
6.1.3 Detector
High-temperature Ni 63 electron capture detector (ECD) with adequate sensitivity to give a signal-to-noise ratio greater than 20 for one picogram of hexachlorobenzene (5.1.2) injected into the column.
The detector shall be operated within its linear range.
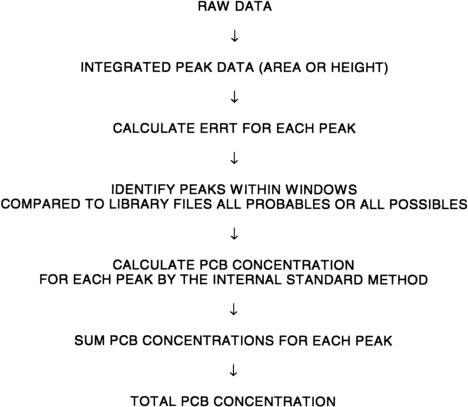
6.2 Data-processing system
Any data-processing system may be used with suitable software that can be programmed to process the operations shown below.
7 Sample
7.1 Sampling
Sampling shall be made in accordance with procedures described in IEC 60475.
To avoid cross-contamination of the samples, it is recommended that all the auxiliary material used (tubing, fittings, corks, connections, etc.) be disposable (single-use) and free from interfering compounds.
7.2 Sample preparation
Only glass or metal apparatus is suitable for sample preparation and the determination except for disposable pipette tips and columns made of plastic. All equipment shall be free from PCBs and interfering substances.
If the samples have a free-water phase, it shall be separated from the oil phase prior to further analysis, for example by centrifuging. Emulsified water perceived as opacity can be removed by adding sodium sulfate in portions and shaking until a clear sample is obtained.
The sample should be homogenized, e.g. by shaking the sample by hand for 3 min. An ultrasonic bath may also be used for this purpose.



