4 Apparatus
4.1 General principle of the method
The liquid sample to be tested, through which a stream of air is bubbled, is maintained for a given period at 120 °C in the presence of solid copper. The resistance to oxidation is evaluated from the amount of total sludge, total acidity and dielectric dissipation factor formed or from the time to develop a given amount of volatile acidity (induction period with air).
4.2 Equipment
4.2.1 Heating arrangement
In order to achieve accurate measurements of the oxidation stability a strict control of the temperature is of high importance. A thermostatically-controlled aluminium alloy block heater or oil bath may be used to maintain the insulating liquid in the desired number of oxidation tubes at the required temperature of 120 °C +/- 0,5 °C (as examples see Figure 1 and Figure 3). This temperature shall be read on a thermometer (see Annex A) inserted in an oxidation tube to within 5 mm from the bottom; this oxidation tube shall be filled with the insulating liquid up to the immersion line of the thermometer and placed in the heating bath.
The temperature of the upper surface of the thermal insulation top shall be maintained at 60 °C +/- 5 °C. Measure this temperature by the use of a thermometer in a drilled aluminium block (see Figure 2). The surfaces of this block, other than that against the upper surface of the heating device, are protected by suitable thermal insulation of nominal 4 mm thickness.
The thermal characteristics of this insulation shall be such as to allow the specified temperatures to be achieved. This block should be placed as near to the holes as practicable and within the area of the upper surface covering the heating device.
When using an aluminum heating block, the oxidation tubes are inserted into the holes to an overall depth of 150 mm. The depth of the holes in the heating part of the block shall be at least 125 mm and short aluminum alloy collars, passing through the insulating cover and surrounding each oxidation tube, will ensure heating over the 150 mm length of the tube.
In the case of oil baths, the oxidation tubes shall be immersed to a depth of 137 mm in the oil and to an overall depth of 150 mm in the bath (see Figure 3).
For both types of heating devices, the height of the oxidation tubes above the upper surface shall be 60 mm and the diameter of the holes shall be just sufficient to allow insertion of the specified tubes. In the case of slackness a 25 mm internal diameter O-ring may be placed around the tube and pressed against the thermal insulation top or inserted into the annular space between the tube and the thermal insulated top. The heating bath should be equipped with supports to hold the absorption tubes.
When in use the heater shall be shielded from direct sunlight and air draughts.
NOTE When oil baths are used, it would be safer to place them in a fume hood.
4.2.2 Test vessels
Test tubes of borosilicate or neutral glass provided with a 24/29 ground joint (see ISO 383), of the following dimensions in mm:
- overall length: 210 +/- 2
- external diameter: 26 +/- 0,5
- wall thickness: 1,4 +/- 0,2
- height of the head: 28 +/- 2
- air inlet tube:
external diameter 5,0 +/- 0,4
wall thickness 0,8 +/- 0,1
The test tube is fitted with a Drechsel head to which is attached the inlet tube which extends to within 2,5 mm +/- 0,5 mm from the bottom and has its end ground at an angle of 60° to the horizontal axis (see Figure 4).
4.2.3 Absorption tubes
These are identical to the test vessels and the distance between the axes of the two tubes shall be 150 mm +/- 50 mm (see Figure 4 and Figure 5). Connections between the test and absorption tubes should be as short as possible, of glass tubing butt-jointed to the vessels by means of short flexible sleeves. Silicone rubber sleeving has been found suitable for this purpose; however, the exposed silicone rubber surface shall be minimized in order to avoid acids from being absorbed by the material. The absorption tubes are mounted outside the heating device.
4.2.4 Filtering crucibles
Gooch-type crucibles with fused-in fritted glass disk according to ISO 4793 porosity 4, designation grade P 16 of, for example, 35 ml capacity.
NOTE Alternatively polymeric membrane filters can be used, provided they are compatible with the insulating liquids and solvents. Suitable membranes consist of a mixture of cellulose esters (cellulose nitrate + cellulose acetate) with the following characteristics:
pore size: 8 µ m;
thickness: 150 µ m;
operating temperature: 120 °C in sterilizer and 75 °C under continuous filtration.
The filtration is improved by impregnating the membrane with a suitable wetting agent (e.g. octyl ethoxylate).
4.2.5 Porcelain vessels
Porcelain crucibles, capacity: 50 ml.
NOTE Alternatively, aluminium foil pans of the same capacity can be used.
4.2.6 Flowmeter
For measuring gas flow-rate a soap bubble flowmeter, a calibrated capillary tube flowmeter or an electronic device can be used.
4.2.7 Timer
For measuring gas flow-rate with soap bubble flowmeter. Subdivisions of the graduation: 0,2 s.
4.2.8 Gas supply
To obtain accurate results it is of high importance to control and maintain the gas flow constant and have a consistent high quality of the gas, this is obtained by the following procedure: gas (oxygen or air according to the method) from a compressed gas cylinder or line, is dried by passing through a scrubber bottle containing concentrated sulphuric acid and then through a tower filled with alternate layers of glass wool and soda lime.
Alternatively drying tubes or a commercial gas purifier may be used.
The dried gas is passed into the oxidation tube via a flow control system which shall be suitable for the specified flow-rate. This may consist of a manifold, connected to the gas-purifying train, with a number of tappings, each provided with a fine-control adjustable needle valve and supplying the gas to one oxidation tube.
The rate of gas flow may be conveniently measured by means of a flowmeter (see 4.2.6). In that case, the difference in level of the liquid in the two limbs of the flowmeter should be sufficiently great to ensure that adequate sensitivity of measurement is obtained over the range of gas flow-rates.
However, any system known to be of equal or greater efficiency can be used.
NOTE A two-stage pressure regulator and a pressure compensator vessel can be helpful to achieve the required accurate regulation of the gas pressure.
4.2.9 Analytical balance
Readability 0,1 mg.
4.2.10 Burette
Volume 10 ml with graduations of 0,01 ml, class A according to ASTM E287.
4.2.11 Volumetric pipette
Volume 25 ml, class A according to ASTM E287.
4.2.12 Volumetric flask
Volume 500 ml, class A according to ASTM E287.
4.2.13 Graduated measuring cylinder
Volume 1 00 ml, class A according to ASTM E287.
4.2.14 Thermometer
A thermometer conforming to the requirements given in Annex A.
4.2.15 Erlenmeyer flask
Erlenmeyer flask, volume 500 ml, with ground glass stopper.
4.3 Reagents
4.3.1 Normal heptane
n-Heptane of analytical grade is to be used.
4.3.2 Alkali blue 6B indicator according to IEC 62021-2
Alkali blue 6B indicator is also known under the chemistry index 42765.
4.3.3 Phenolphthalein indicator
1 g of phenolphthalein per 100 ml of azeotropic ethanol (about 5 % water). Alternatively isopropanol containing 5 % of water may be used.
NOTE Phenolphthalein fades rather quickly when exposed to strong direct light, should a faint tint be observed, it is suggested that a few more drops of indicator are added.
4.3.4 Potassium hydroxide according to IEC 62021-2
0,05 mol/l alcoholic solution.
4.3.5 Oxidant gas
Synthetic air or air from compressed air line, free of hydrocarbons.
4.3.6 Acetone
Acetone of analytical grade is to be used.
4.4 Cleaning of test vessels
The test and the absorption tubes shall be chemically cleaned. Wash with acetone followed by distilled or deionized water.
Drain and then soak in 95 % to 97 % sulphuric acid for a minimum of 16 h. Drain and complete removal of acid by washing, first with tap water, then with distilled or deionized water. Dry the tubes in an air oven at 1 05 °C for at least 3 h, and then allow cooling to room temperature in a desiccator or a dry cabinet in which they are kept ready for use. Other cleaning methods giving the same cleanliness result can be used.
4.5 Catalyst
The solid copper used as oxidation catalyst consists of a wire of soft electrolytic copper, of diameter between 1 mm and 2 mm (of such a length as to give a surface area of 28,6 cm2 +/- 0,3 cm2). To get accurate results it is of high importance that the copper surface is properly prepared according to the following procedure:
- Immediately before use, the requisite length of copper wire is cleaned with P220 grade silicon carbide abrasive cloth (ISO 6344-1). All traces of abrasive are removed with a lintless filter paper and then with a dry, lintless cloth.
- Roll the wire into a spiral of approximately 2 cm external diameter and 5 cm long.
- The spiral is thoroughly cleaned by dipping it into normal heptane, then dried in air and immediately introduced into the test vessel.
To avoid contamination, the prepared coil shall be handled only with tweezers. The copper wire shall not be re-used.
4.6 Insulating liquid sample conditioning
Liquid to be tested shall be filtered through a previously dried (1 h at 105 °C) fritted glass filter (ISO 4793, porosity 4, designation grade P16) or on membrane filters of 8 µm to remove traces of sediment, fiber and excess water. The first 25 ml of filtrate should be discarded.
4.7 Preparation of the test
Adjust the heating bath to maintain the insulating liquid in all oxidation tubes at the required temperature of 120 °C +/- 0,5 °C (thermometer complying with the requirements of Annex A).
Weigh in each 25 g +/- 0,1 g of insulating liquid into three oxidation tubes and insert the catalyst coil previously prepared as described in 4.5. At least three oxidation tubes are required to be able to measure the DDF (minimum two tubes) and sludge/oil acid number (one tube). Insert the Drechsel head and place the tube into the heater using a rubber O-ring if necessary to close the gap between the tube and the thermal insulated top.
Pour 25 ml of distilled water into one absorption tube. Insert the Drechsel head and connect to the corresponding oxidation tube (see Figure 4 ).
Adjust the air flow to deliver 0,150 l/h +/- 0,015 l/h measured by means of the flowmeter (see 4.2.6) connected to the outlet end of the absorption tube (see Figure 5).
Oxidize the insulating liquid while maintaining its temperature at 120 °C +/- 0,5 °C and an air flow-rate of 0,150 l/h +/- 0,015 l/h.
Check air flow and temperature daily.
4.8 Determinations on the oxidized insulating liquid
4.8.1 Sludge formation
The sludge shall be precipitated by adhering strictly to the procedure described below.
The sample of 25 g of artificially aged insulating liquid is cooled in the dark for 1 h, and is then poured into an Erlenmeyer flask.
Use 300 ml normal heptane in successive fractions to rinse out the insulating liquid adhering to the test tube, copper spiral and gas lead-in tube and add the washings to the insulating liquid in the flask.
The mixture is then allowed to stand in the dark for 24 h, at a temperature of 20 °C +/- 5 °C, before filtering through a filter crucible (or membrane filter) previously dried to constant mass.
At the start of filtering only a small pressure drop should be used, to prevent the sludge passing through the filter. Cloudy filtrates should be passed through a second time.
All traces of insulating liquid shall be removed by repeated washing of the sludge with normal heptane. The total volume of the normal heptane used for the washing of the sludge shall be 150 ml. The filter containing the sludge is dried at 105 °C to constant mass.
Sludge adhering to the catalyst, to the test tube, and to the gas lead-in tube is transferred, by dissolving it in small quantities of acetone (a total of 30 ml), to a tared porcelain vessel (or aluminum foil pan). It is then dried at 105 °C, after the evaporation of the acetone, to constant mass. The mass of the residue is added to that of the sludge obtained by precipitation with normal heptane.
The total sludge is expressed as a percentage of the initial weight of the insulating liquid.
4.8.2 Soluble acidity (SA)
The heptane solution obtained after filtering off the sludge is collected in a 500 ml volumetric flask and made up to mark with normal heptane. Three determinations of the neutralization value are made on 100 ml samples of the heptane insulating liquid solution.
The determination is carried out with 1 00 ml of the heptane solution according to IEC 62021-2.
Calculate (Formula 1) the soluble acidity (SA), in milligrams of potassium hydroxide per gram of insulating liquid, as follows:
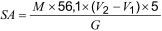
where:
M is the molarity of the alcoholic potassium hydroxide solution;
V2 is the volume of alcoholic potassium hydroxide solution in ml necessary to titrate the normal heptane insulating liquid solution;
V1 is the volume of alcoholic potassium hydroxide solution in ml necessary to titrate 100 ml of normal heptane with an alkali blue 6B indicator solution according to IEC 62021-2;
G is the mass of insulating liquid, in grams.
NOTE The potentiometric method for the determination of soluble acidity (IEC 62021-1) can be used alternatively if proven to deliver the same result.
4.8.3 Volatile acidity (VA)
Volatile acidity is a measurement of the amount of oxidation products collected in the absorption tube. Measurements are performed at the end of the test duration by neutralizing the acids by titration with a 0,05 mol/l alcoholic potassium hydroxide solution using phenolphthalein as indicator.
Titration is determined as follows:
- disconnect the absorption tube;
- titrate the volatile acidity directly in the absorption tube with a 0,05 M alcoholic potassium hydroxide solution using a few drops of the solution of phenolphthalein as indicator;
- the volatile acidity (VA) is calculated (Formula 2) in milligrams of potassium hydroxide per gram of insulating liquid, as follows:
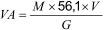
where:
M is the molarity of the alcoholic potassium hydroxide solution;
V is the number of ml of the alcoholic potassium hydroxide solution used in the titration;
G is the mass of oil, in grams.
4.8.4 Total acidity (TA)
Total acidity, in milligrams of potassium hydroxide per gram of insulating liquid, is calculated as the sum of the volatile and soluble acidities (Formula 3):
TA = SA + VA
4.8.5 Dielectric dissipation factor (DDF)
Carried out according to IEC 60247 with the following additions.
Prepare separately oxidized insulating liquid as follows: after removing the test tubes from the oxidation bath, close the tubes and store them for 24 h at room temperature (20 °C +/- 5 °C). During this period the sample will cool down and insoluble sludge will settle to the bottom of the test tubes. Decant the insulating liquid without agitation into a cleaned test cell ensuring that the sludge remains undisturbed and is not transferred into the test cell. Only approximately 80 % of the insulating liquid shall be transferred; the remaining sludge/insulating liquid stays in the test tube and shall not be used for the DDF determination.
4.8.6 Oxidation rate with air
A graphical representation of the oxidation rate over the entire period can be obtained by titrating volatile acidity daily (or at other suitable time interval) and plotting the cumulated results against time.
4.8.7 Induction period with air (IP with air) (optional)
The induction period is determined by reading the time corresponding to 0,10 mg KOH/g volatile acidity in the case of mineral oils. In the case of ester insulating liquids higher acidity values may be applicable and shall be defined.
4.9 Report
The report shall include the following:
- IEC 61125;
- test duration;
- total acidity (TA), reported to the nearest 0,01 mg KOH/g;
- sludge, reported to the nearest 0,01 % by mass;
- dielectric dissipation factor, reported to the nearest 0,001 .
4.10 Precision
4.10.1 General
The following precision values were obtained on uninhibited mineral insulating oils (see Note in 4.10.3) in a 164 h test.
4.10.2 Repeatability (r) (95 % confidence)
Duplicate results obtained at the same time by the same operator should be considered suspect if their difference, when expressed as a percentage of their mean, is higher than the amount given in Table 1.
4.10.3 Reproducibility (R) (95 % confidence)
The results (see Note below) submitted by each of two laboratories should be considered suspect if their difference, when expressed as a percentage of their mean, is more than the amount given in Table 1.

NOTE The precision has not been established for inhibited mineral insulating oils. However, both repeatability and reproducibility are dependent upon the precision of the induction period and the slope of the oxidation rate curve. A flat curve will give a precision comparable to that of the uninhibited mineral insulating oils.
A steep slope can give poor precision if the break point of the oil occurs near the termination of the test. The oxidation rate curve can be obtained by plotting the volatile acidity against time, as for the induction period.



