10. Procedure for Soluble Materials, Either Liquid or Solid
10.1 Pipet 25 to 50 mL of the selected solvent into the titration cell. Titrate the water in the solvent with KF reagent according to the instrument manufacturer's instructions. The KF reagent that is used should be of appropriate titer as determined by the amount of water anticipated in the sample (see 10.2).
10.2 Weigh or pipet a sample containing an anticipated water content that will give a fast and accurate titration. KF instrument operation manuals typically list suggested sample sizes, however, Table 1 also can be used as a guideline for sample sizes of the three most common titrant titers. Keep in mind that very small sample amounts may be difficult to accurately weigh and transfer, whereas, very large sample amounts may result in incomplete miscibility with the chosen solvent.
NOTE 14 - The KF technique described here is sometimes referred to as the "one component" method because all the reagents are in the titrant, and the solvent is used basically as a medium to dissolve the sample. There is also a "two component" KF volumetric titration in which the titrant contains the usual reagents, but the solvent also contains sulfur dioxide and a base. There are advantages to the two component system since strongly basic or acid samples can overcome the buffering capacity of the single component system and cause the pH of the reaction mixture to shift from the optimum range. The two component system provides initial sample buffering capacity in the solvent which may provide a faster reaction time. Rapid end point determination also can provide more accurate measurement of trace water concentrations. Two component reagents, however, are more susceptible to side reaction from noncomplexed sulfur dioxide than single component systems (5).
NOTE 15 - The range of water indicated is for macro titrations. Considerably smaller amounts of water can be determined precisely on a micro scale. For example, less than 300 µg of water were titrated in 1-mL samples of benzene by a micro amperometric technique (6).
10.3 Calculation - Calculate the water content of the sample as follows:
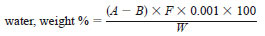
where:
A = millilitres of reagent required for titration of the sample,
B = millilitres of reagent required to titrate solvent blank,
F = water equivalent, in milligrams of water per millilitre of KF reagent, and
W = grams of sample.
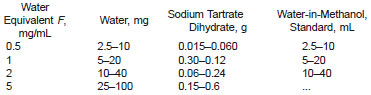
11. Standardization of Karl Fischer Reagent
11.1 Standardize the KF reagent daily or as necessary using the amounts of water, sodium tartrate dihydrate, or water-in-methanol shown below:
11.2 Pipet 25 to 50 mL of methanol or appropriate solvent to a clean, dry titration cell and pretitrate according to the instrument manufacturer's instructions.
11.3 Transfer the selected standard to the pretitrated solvent.
11.3.1 Weigh, to the nearest 0.0001 g, the indicated amount of water from a suitable weighing pipet, hypodermic syringe, or other device, or
11.3.2 Transfer the weighed sodium tartrate dihydrate by means of a dry spatula, dipping the spatula into the alcohol to ensure removal of any adhering tartrate (Note 16), or
11.3.3 Use a hypodermic syringe of suitable capacity to transfer the standard water-in-methanol solution to the titration flask.
NOTE 16 - To facilitate transferral of the tartrate to vessels having constricted openings or narrow necks, a spatula with the tip bent at a right angle to the handle is satisfactory. If the tartrate is used for standardizing Karl Fischer reagent for use with samples containing more than 1 % water, a bias may exist which has been described in Ref (7).
11.4 Titrate with KF reagent to the instrument manufacturer's instructions.
11.5 Calculation - Calculate the water equivalent, E, of the KF reagent, in milligrams per millilitre, as follows:
Water as Standard:
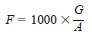
Water-in-Methanol as Standard:

Sodium Tartrate Dihydrate as Standard:
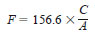
where:
G = grams of water used,
C = grams of sodium tartrate dihydrate used,
A = millilitres of reagent required for titration of the standard,
D = millilitres of water-in-methanol standard required, and
E = milligrams of water per millilitre in the water-in-methanol standard.

