12. Quality Control Checks
12.1 Confirm the performance of the equipment or the procedure each day it is in use, by analyzing a quality control (QC) sample. It is advisable to analyze additional QC samples as appropriate, such as at the end of a batch of samples or after a fixed number of samples to ensure the quality of the results. Analysis of results from these QC samples can be carried out using control chart techniques. When the result of a test on a QC sample exceeds the control limits of the laboratory, corrective action, such as instrument recalibration may be required. An ample supply of QC sample material shall be available for the intended period of use, and shall be homogeneous and stable under the anticipated storage conditions. If possible, the QC sample shall be representative of samples typically analyzed and the average value and control limits of the QC sample shall be determined prior to monitoring the measurements process. The precision for the QC sample must be compared against that given in the Precision and Bias section of this test method in order to verify that the instrument is functioning correctly.
NOTE 16 - Because the acid and base numbers can vary while the QC sample is in storage, when an out-of-control situation arises, the stability of the QC sample can be a source of the error.
13. Calculation
13.1 Calculate the acid number as follows:
Acid number, mg of KOH/g = [(A - B)M x 56.1]/W
where:
A = KOH solution required for titration of the sample (9.2), mL,
B = KOH solution required for titration of the blank (9.3), mL,
M = molarity of the KOH solution, and
W = sample used, g.
13.2 Calculate the strong-acid number as follows:
13.2.1 If the blank titration is made with acid:
Strong - acid number, mg of KOH/g = [(CM + Dm) x 56.1]/W
where:
C = KOH solution required to titrate the water extract (11.1), mL,
M = molarity of KOH solution,
D = HCl solution required to titrate the blank solution (11.2), mL,
m = molarity of the HCl solution, and
W = sample used, g.
13.2.2 If the blank titration is made with base:
Strong - acid number, mg of KOH/g = [(C - D)M x 56.1]/W
where:
C = KOH solution required to titrate the water extract (11.1), mL,
D = KOH solution required to titrate the blank solution (11.2), mL,
M = molarity of the KOH solution, and
W = sample used, g.
13.3 Calculate the base number as follows:
base number, mg of KOH/g = [(Em + FM) x 56.1]/W
where:
E = HCl solution required for titration of the sample (Section 10), mL,
m = molarity of the HCl solution,
F = KOH required for titration of the acid number blank, mL,
M = molarity of the KOH solution, and
W = sample used, g.
14. Report
14.1 Report the result as acid number, strong acid number, or base number as follows:
Acid number (D974) = (result)
Strong acid number (D974) = (result)
Base number (D974) = (result)
14.2 Report the acid or base number values as follows:

15. Precision and Bias
15.1 Precision - This precision section applies only to new, light-colored, straight mineral oils and new and used inhibited steam turbine oils. Insufficient data are available on other oils coming within the scope of this test method so that no precision is given for such oils.
15.1.1 Repeatability - The difference between two test results, obtained by the same operator with the same apparatus under constant operating conditions on identical test material, would in the long run, in the normal and correct operation of the test method, exceed the following values only in one case in twenty:

15.1.2 Reproducibility - The difference between two single and independent results obtained by different operators working in different laboratories on identical test material would, in the long run, in the normal and correct operation of the test method, exceed the following values only in one case in twenty:
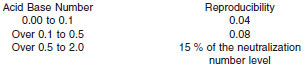
NOTE 17 - These precision values do not apply to oils that are so highly colored as to obscure the end point color change.
NOTE 18 - For precision applicable to electrical insulating liquids, refer to Guide D117.
NOTE 19 - The precision statements were based on the use of manual burets only. The user is cautioned that the precision statements may or may not be applicable to titrations performed with the use of automated burets, since no interlaboratory study has been conducted to date to statistically evaluate results determined by both techniques.
15.2 Bias - The procedures in this test method have no bias because the acid and base values can be defined only in the terms of the test method.
16. Keywords
16.1 acid number; base number; color indication titration; petroleum products

