6. Apparatus
6.1 Burets (with the following dimensions):
50-mL buret graduated in 0.1-mL subdivisions
10-mL buret graduated in 0.05-mL or smaller subdivisions
5-mL with 0.02-mL subdivisions
NOTE 4 - An automated buret capable of delivering titrant amounts in 0.05-mL or smaller increments can be used but the stated precision data were obtained using manual burets only.
7. Reagents
7.1 Purity of Reagents - Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available.4 Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination.
7.2 Purity of Water - References to water shall be understood to mean reagent water that meets the requirements of either Type I, II, or III of Specification D1193.
7.3 Isopropyl Alcohol, anhydrous (less than 0.9 % water). (Warning - Flammable.)
7.4 Hydrochloric Acid Solution, Standard Alcoholic - (0.1 M) - Mix 9 mL of concentrated hydrochloric acid (Warning - Corrosive, fumes cause irritation) (HCl, sp gr 1.19) with 1000 mL of anhydrous isopropyl alcohol (2-propanol) (Warning - See 7.3). Standardize frequently enough to detect molarity changes of 0.0005 (Note 6), preferably by electrometric titration of approximately 8 mL (accurately measured) of the 0.1 M alcoholic KOH solution diluted with 125 mL of carbon dioxide-free water. When an electrometric titration is used for the standardization, the end point shall be a well-defined inflection point closest to the cell voltage for the acidic buffer solution. When a colorimetric titration is used for the standardization, titrate to the first stable appearance of the orange color with methyl orange indicator.
NOTE 5 - Commercially available reagents may be used in place of the laboratory preparations when they are certified to be in accordance with 7.1.
NOTE 6 - To simplify calculations, both the standard KOH and HCl solutions can be adjusted so that 1.00 mL is equivalent to 5.00 mg of KOH. Sodium hydroxide (NaOH) and sulfuric acid (H2SO4) can be substituted for KOH and HCl, respectively.
7.5 Methyl Orange Indicator Solution - Dissolve 0.1 g of methyl orange in 100 mL of water.
7.6 p-Naphtholbenzein Indicator Solution - The p-naphtholbenzein shall meet the specifications given in Annex A1. Prepare a solution of p-naphtholbenzein in titration solvent equal to 10 more or less 0.01 g/L.
7.7 Potassium Hydroxide Solution, Standard Alcoholic (0.1 M) - Add 6 g of solid KOH (Warning - Highly corrosive to all body tissue) to approximately 1 L of anhydrous isopropyl alcohol (containing less than 0.9 % water) in a 2-L Erlenmeyer flask. Boil the mixture gently for 10 to 15 min, stirring to prevent the solids from forming a cake on the bottom. Add at least 2 g of barium hydroxide (Ba(OH)2) (Warning - Poisonous if ingested, strongly alkaline, causes severe irritation producing dermatitis) and again boil gently for 5 to 10 min. Cool to room temperature, allow to stand for several hours, and filter the supernatant liquid through a fine sintered-glass or porcelain filtering funnel; avoid unnecessary exposure to carbon dioxide (CO2) during filtration. Store the solution in a chemically resistant dispensing bottle out of contact with cork, rubber, or saponifiable stopcock lubricant and protected by a guard tube containing soda lime or soda nonfibrous silicate absorbent (Ascarite, Carbosorb, or Indecarb).
7.7.1 Standardization of Potassium Hydroxide Solution - Standardize frequently enough to detect changes of 0.0005 M. One way to do this is as follows: Weigh, to the nearest 0.1 mg approximately 0.2 g of potassium acid phthalate, which has been dried for at least 1 h at 110 more or less 1°C and dissolve in 40 more or less 1 mL of water, free of CO2. Titrate with the potassium hydroxide alcoholic solution to either of the following end points: (1) When the titration is electrometric, titrate to a well-defined inflection point at the voltage that corresponds to the voltage of the basic buffer solution, or (2) When titration is colorimetric, add six drops of phenolphthalein indicator solution and titrate to the appearance of a permanent pink color. Perform the blank titration on the water used to dissolve the potassium acid phthalate. Calculate the molarity using the following equation:
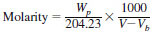
where:
Wp = weight of the potassium acid phthalate, g,
204.23 = molecular weight of the potassium acid phthalate,
V = volume of titrant used to titrate the salt to the specific end point, mL, and
Vb = volume of titrant used to titrate the blank, mL.
7.7.2 Prepare a 0.1 more or less 0.01 m% solution of phenolphthalein by dissolving pure solid phenolphthalein in a 1:1 mixture of water, free of CO2, and ethanol.
NOTE 7 - Commercially available reagents may be used in place of the laboratory preparations.
NOTE 8 - Because of the relatively large coefficient of cubic expansion of organic liquids, such as isopropyl alcohol, the standard alcoholic solutions should be standardized at temperatures close to those employed in the titrations of samples.
7.8 Titration Solvent - Prepare by mixing toluene, water, and anhydrous isopropyl alcohol in the ratio 100 : 1 : 99.
8. Preparation of Used Oil Samples
8.1 Strict observance of the sampling procedure described in 8.2 is necessary, since the sediment itself is acidic or basic or has adsorbed acidic or basic material from the sample. Failure to obtain a representative sample causes serious errors.
8.2 Heat the sample (Note 9) of used oil to 60 more or less 5°C in the original container and agitate until all sediment is homogeneously suspended in the oil (Note 10). If the original container is of opaque material, or if it is more than three-fourths full, transfer the entire sample to a clear glass bottle having a capacity at least one third greater than the volume of the sample, and transfer all traces of sediment from the original container to the bottle by violent agitation of portions of the sample in the original container. After complete suspension of all sediment, strain the sample or a convenient aliquot through a 100-mesh screen for the removal of large contaminating particles (Note 9).
NOTE 9 - When samples are visibly free of sediment, the heating procedure described in 8.2 may be omitted. When samples are visibly free of sediment, the straining procedure may also be omitted.
NOTE 10 - As used oil can change appreciably in storage, samples should be tested as soon as possible after removal from the lubricating system and the dates of sampling and testing should be noted.

