PROCEDURE B (VOLUMETRIC)
17. Reagents
17.1 Purity of Reagents - Use reagent grade chemicals in all tests. Unless otherwise indicated, all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society where such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination.
17.2 Purity of Water - Unless otherwise indicated, references to water shall mean reagent water as defined by Type II and III of Specification D1193.
17.3 Karl Fischer Reagents - Pyridine-free formulations are preferred by most KF instrument manufacturers for use with their equipment. These reagents are less toxic, less odorous, and more stable than those containing pyridine. The use of pyridine-free reagents is recommended.
17.3.1 One-component Karl Fischer Titrant - Typically consists of a mixture of an organic base, sulfur dioxide and iodine dissolved in a solvent such as diethyleneglycol monoethyl ether. Reagents with titers of H2O at 2.0 mg/mL are recommended for this method. Reagents with titers of H2O at 5.0 mg/mL may also be used. See Note 4.
17.3.2 Two-component Karl Fischer Titrant and Solvent - Titrant typically consists of iodine and methanol or ethanol. Contains sulfur dioxide and organic base in anhydrous methanol or ethanol. See Note 4.
NOTE 4 - The "one-component" KF volumetric titrant contains all the necessary reagents for titration of water and the solvent is used basically as a medium to dissolve the sample. The "two-component" KF volumetric titrant contains iodine and methanol or ethanol and the solvent contains sulfur dioxide and a base. The one component system is simple and allows for flexibility in solvent choice. However, because all of the necessary chemicals for titration are present, the titer decreases significantly over time. The two-component system will remain stable for longer periods of time and can provide more accurate measurement of trace water concentrations. Two-component reagents, however, are more susceptible to side reaction from non-complexed sulfur dioxide than single-component systems.
17.4 Solvents:
17.4.1 Methanol, max 0.15 % water, in accordance with Specification D1152. (See Note 5.)
17.4.2 Denatured Ethanol, max 0.15 % water.
NOTE 5 - Solvents with low water content will consume less Karl Fischer reagents during the pre-titration process. Cosolvents are acceptable so long as solubility is not compromised.
17.5 Water Standard - Solvent mixture with precisely determined water content intended for calibrating volumetric KF reagents. Standards should come complete with a Certificate of Analysis. Standards containing 1.0 % water are recommended for this test method. High purity water may also be used for titrant standardization.
18. Preparation (Standardization of the Karl Fischer Titrant)
18.1 Standardize the KF reagent using high purity water or water standard daily. Standardization may be performed less frequently when using a daily check standard to verify KF reagent titer. Standard recovery should be within 97 % to 103 % of the certified value. Table 2 shows the suggested sample size that will require 2 mL to 10 mL of titrant for titration of the sample. Approximately 0.4 g to 1 g of 1.0 % water standard is recommended for standardization of a titrant with an expected titer of 2.0 mg/mL. Although pure water may be used to standardize KF titrants, weighing errors increase due to the small sample size required.
18.2 Prepare the dispensing buret and any tubing to ensure dispensed quantities are accurately measured. Care should be taken to ensure air bubbles are not dispensed during the titration.
18.3 Add approximately 25 mL to 50 mL or an appropriate amount of methanol or solvent ifusing two-component reagent to cover the electrode tip. See Note 6.
NOTE 6 - The solvent volume should cover the double platinum electrode tip during stirring. Stir speed should be controlled to ensure proper mixing but should not create a vortex funnel.
18.4 Pre-titrate with KF reagent according to the instrument manufacturer's instructions.
18.5 Transfer standard to the pretitrated solvent.
18.5.1 Assemble a dry gas-tight 5 mL or 10 mL syringe and needle with suitable capacity to make three to five standardization titrations. See 7.2.
18.5.2 Rinse the syringe assembly by withdrawing approximately 0.25 mL of standard into the syringe. Eject to waste in an appropriate waste container. Repeat rinsing the syringe with standard two additional times to assure a representative sample and remove any residual moisture from the syringe.
18.5.3 Withdraw standard into the syringe according to the expected water content and titrant concentrations. Invert the syringe and eject to remove any air. Wipe any excess liquid from the needle. Obtain the mass of syringe and sample to +/- 0.1 mg (W1). See Table 2 and Note 4.
18.5.4 With the analyzer stabilized, carefully insert the needle of the sample syringe through the septum and below the level of solution in the titration chamber. Inject the sample slowly and carefully into the titration solution and begin titration. Withdraw the syringe needle and weigh to the nearest +/- 0.1 mg (W2) to determine the exact sample mass. Keep in mind that very small sample amounts can be difficult to accurately weigh and transfer.
18.6 Allow the titration to proceed until the end-point is indicated. After repeated injections, the level of solvent may need to be reduced or replaced. Follow the manufacturer's instructions. Discard solution and replace with fresh titration solution if a stable reading cannot be obtained.
18.7 End Point Detection - End point is detected automatically and the water content is calculated based on the sample mass entered. During the titration a small constant polarization current is applied to the double platinum electrode and the voltage required to maintain this current is measured. When water is present in the titration vessel, the voltage required is high. Once there is a slight excess of iodine, the voltage required is reduced. This large change indicates the titration end point.
18.8 Record the volume of titrant dispensed to reach the end point.
19. Calculation for Standardization of the KF Titrant 19.1 The water equivalent (titer) of the KF reagent, in milligrams per milliliter, can be manually calculated using Eq 5 if high purity water is used or Eq 6 if a certified water standard is used. Most instruments are equipped to provide a calculated result based upon the entered sample size.
High Purity Water:
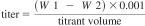
Water Standard:
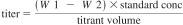
where:
titrant volume = milliliters of titrant required for titration of the sample,
standard conc = water content of standard, mg/g,
W1 = mass of sample and syringe before injection, g, and
W2 = mass of sample and syringe after injection, g.
20. Procedure
20.1 Prepare the dispensing buret and any tubing to ensure dispensed quantities are accurately measured. Ensure air bubbles are not dispensed during the titration.
20.2 Add 25 mL to 50 mL of the selected solvent into the titration cell. The volume of solvent should be added such that the metal portion of the electrode is completely submerged. See Note 7.
NOTE 7 - If the titration vessel contains KF solvent that has been pre-titrated for standardization, there is no need to replace solvent. Proceed to 20.3.
20.3 Pre-titrate the water in the solvent with KF reagent according to the instrument manufacturer's instructions. The KF reagent that is used should be of appropriate titer as determined by the amount of water anticipated in the sample.
20.4 Transfer the sample to titration vessel.
20.4.1 Assemble a dry gas-tight syringe and needle with suitable capacity. See 7.2.
20.4.2 Rinse the syringe assembly by withdrawing approximately 0.5 mL of sample into the syringe. Eject to waste in an appropriate waste container. Repeat rinsing the syringe with sample two additional times to assure a representative sample.
20.4.3 Withdraw sample into the syringe according to the expected water content. Invert the syringe and eject to remove any air. Wipe any excess liquid from the needle. Obtain the weight of syringe and sample to +/- 0.1 mg (W1). KF instrument operation manuals typically list suggested sample sizes, however, Table 3 may be used as a guideline for sample sizes based on titrant concentrations. Keep in mind that very small sample amounts can be difficult to accurately weigh and transfer.
20.4.4 With the analyzer stabilized, carefully insert the needle of the sample syringe through the septum and below the level of solution in the titration chamber. Inject the sample slowly and carefully into the titration solution and begin titration. Withdraw the syringe needle and weigh to the nearest +/- 0.1 mg (W2) to determine the exact sample mass.
20.4.5 Allow the titration to proceed until the end-point is indicated. After repeated injections, the level of solvent may need to be reduced or replaced. Follow the manufacturer's instructions. Discard the solution and replace with fresh titration solution if a stable reading cannot be obtained.
20.5 End Point Detection - End point is detected automatically and the water content is calculated based on the sample weight entered. During the titration a small constant polarization current is applied to the double platinum electrode and the voltage required to maintain this current is measured. When water is present in the titration vessel the voltage required is high. Once there is a slight excess of iodine, the voltage required is reduced. This indicates the titration end point.
20.6 Record the volume of titrant dispensed to reach the end point.
21. Calculation of Sample Content
21.1 The water content may be manually calculated as percent by mass using Eq 7 or as percent by volume using Eq 8. Most instruments are equipped to provide a calculated result based upon the entered sample size.
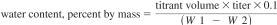
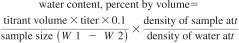
where:
titrant volume = amount required for titration of the sample, mL,
titer = equivalent amount of water in KF reagent, mg/mL,
t = test temperature, °C,
W1 = mass of sample and syringe before injection, g,
W2 = mass of sample and syringe after injection, g, and
0.1 factor = conversion factor, %.
22. Verification of Calibration and Quality Control 22.1 Autotitrators vary in calibration procedures by manufacturer. Consult the operating manual for the autotitrator in use. Stable, prepackaged quality control (QC) water standards are commercially available with 0.1 % by mass and 1 % by mass water content for this purpose. It is desirable to verify calibration with a standard solution that approximates the same range of water expected to be in the samples.
22.2 It is recommended that a control chart measuring a QC standard sample be established and maintained according to generally accepted guidelines. Practice D6299 can be used for this purpose. Measure the control sample each day a sample(s) is tested. If the measured value exceeds +/- 5 % of the known amount, take appropriate action before proceeding with the sample test.
23. Report
23.1 Report the percentage of water to the nearest 0.01 % by mass. Alternatively, report the percentage of water to the nearest 0.01 % by volume.
23.2 Report the water concentration in one of the defined units as obtained by Test Method D7923, Procedure B.
24. Precision and Bias
24.1 The statistical precision of this procedure, as determined by statistical examination of the 2015 interlaboratory test results, obtained from 8 laboratories on 14 samples, is as follows:
24.1.1 Repeatability - The difference between successive results obtained by the same operator with the same apparatus under constant operating conditions on identical test material, would in the long run, in the normal and correct operation of the test method, exceed the following values in one case in twenty.
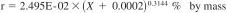
where:
X = the calculated result for percentage of water expressed as percent by mass.
24.1.2 Reproducibility - The difference between two single and independent results, obtained by different operators working in different laboratories on identical material, would be in the long run, in the normal and correct operation of the test method, exceed the following values only in one case in twenty.
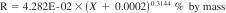
where:
X = the calculated result for percentage of water expressed as percent by mass.
NOTE 8 - The data in Table 4 shows repeatabilities and reproducibilities for water values obtained using the formulas given in 24.1.1 and 24.1.2.
24.1.3 The precision statement was determined through statistical examination of 14 materials with blind duplicates from eight laboratories. The materials included one sample of anhydrous ethanol, six ethanol blends ranging from a nominal 5 % to 85 % ethanol, three samples of denatured fuel ethanol, two samples of gasoline, and two samples of hydrous ethanol. Water contents of the samples ranged from 0.005 % by mass to 5.41 % by mass by Procedure B.
24.2 Bias - The bias of this test method has not been determined since no acceptable reference material has been identified.
24.3 Relative Bias - A relative bias assessment of Procedure B versus Procedure A of this test method for the determination of water by Karl Fischer titration was performed in accordance with the requirements of Practice D6708 with a successful outcome (type A4).
24.3.1 The degree of agreement between results from Procedure B (Volumetric Titration) and Procedure A (Coulometric Titration) can be further improved by applying the correction equation listed below (Research Report RR:D02-1839). Sample-specific bias, as defined in Practice D6708, was observed for some samples after applying the bias-correction. The bias was determined from eleven common samples of ethanol and hydrocarbon blends used in the precision study for the two procedures. The samples had water content ranging from 0.002 % by mass to 1.63 % by mass by Procedure A and 0.005 % by mass to 1.61 % by mass by Procedure B.
Correction Equation:
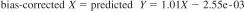
where:
X = result by Procedure B (Volumetric Titration), % by mass,
bias-corrected X = predicted Y, % by mass, and
predicted Y = result that would have been obtained by Procedure A (Coulometric Titration) on the same sample.
24.3.2 Differences between bias-corrected results from Procedure B and Procedure A from this method, for the sample types and property ranges studied, are expected to exceed the following between methods reproducibility (RXY), as defined in Practice D6708, about 5 % of the time. As a consequence of sample-specific biases, RXYmay exceed the reproducibility for Procedure B, or the reproducibility for Procedure A, or both. Users intending to use Procedure B as a predictor of Procedure A, or vice versa, are advised to assess the required degree of prediction agreement relative to the estimated RXY to determine the fitness-for use of the prediction.
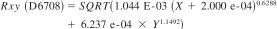
25. Keywords
25.1 coulometric; ethanol; gasoline; hydrocarbon; Karl Fischer; pyridine-free; volumetric; water content

