PROCEDURE A (COULOMETRIC)
10. Reagents
10.1 Purity of Reagents - Unless otherwise indicated, it is intended that all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination.
10.2 Purity of Water - Unless otherwise indicated, reference to water shall be understood to mean Type II or Type III reagent water, conforming to Specification D1193, or better.
10.3 Karl Fischer Reagents - Commercial coulometric KF reagents and reagent systems of various types are available for use with autotitrators for water determination. Traditionally, pyridine was the organic base used in KF reagents. Pyridine-free formulations are available and are preferred by most KF instrument manufacturers for use with their equipment. The pyridine-free reagents are less toxic, less odorous, and more stable than those containing pyridine. The use of pyridine-free reagents is recommended whenever possible. Coulometric titrations normally require two reagent solutions: an anolyte and a catholyte or generator solution. However, with the use of an integrated or diaphragm-less cell, a single solution that contains all of the reagents needed for a KF titration may be used.
10.3.1 Catholyte solution, contains ammonium salts and methanol.
10.3.2 Anolyte solution, contains iodide, sulfur dioxide and imidazole buffer in a suitable solvent.
10.3.3 One component solution, iodide, sulfur dioxide, imidazole buffer, and bases in a suitable solvent. This solution may be used as the only solution in a coulometric system with a diaphragm-less generator cell or as the anolyte solution in a diaphragm cell if specified by the manufacturer.
10.3.4 Water Standards, 0.1 % by mass and 1 % by mass, commercially prepared in organic solvent recommended for this method.
11. Preparation of Apparatus
11.1 Clean, dry, and assemble the titration chamber as directed in the manufacturer's instructions. Care should be taken to ensure the vessel is sealed from atmospheric moisture. Replace the desiccant if saturated. Connect the leads from the sensing and generator electrodes to the titrator.
11.2 Add catholyte solution (10.3.1) to the generator electrode assembly and reseal the vessel according to manufacturer instructions.
11.3 Fill the anode reservoir with anolyte solution (10.3.2) as directed by the manufacturer. The level of the catholyte solution in the inner chamber shall be maintained slightly below the level of the anolyte solution to prevent backflow contamination of the titration (anolyte) solution. As samples are analyzed, the volume of the anolyte will increase. This may slow reactivity of the catholyte due to increased pressure across the membrane. A portion of the anolyte solution may have to be removed periodically to maintain the correct level. (Note 1)
NOTE 1 - A coulometric system with a diaphragm-less generator electrode should be filled with the appropriate one component solution.
11.4 Agitate the titration solution by gently swirling the titration chamber to remove any residual moisture from the walls. Allow the solution to stir until inner atmosphere moisture is removed and the baseline has been established.
12. Verification of Calibration and Quality Control
12.1 Autotitrators vary in calibration procedures by manufacturer. Consult the operating manual for the autotitrator in use. Stable, prepackaged quality control (QC) water standards are commercially available with 0.1 % by mass and 1 % by mass water content for this purpose. It is desirable to verify calibration with a standard solution that approximates the same range of water expected to be in the samples.
12.2 It is recommended that a control chart measuring a QC standard sample be established and maintained according to generally accepted guidelines. Practice D6299 may be used for this purpose. Measure the control sample each day a sample(s) is tested. If the measured value exceeds +/- 5 % of the known amount, take appropriate action before proceeding with the sample test (see Note 2).
NOTE 2 - This may require replacing the reagent solutions.
13. Procedure
13.1 Assemble a dry syringe and needle. Withdraw 1 mL to 2 mL of the sample into the syringe and discard the contents into a waste container. Repeat rinsing the syringe with sample two additional times to assure a representative sample and remove any residual moisture from the syringe. During sampling, minimize sample exposure to atmospheric moisture. Using the following table as a guide, withdraw the proper amount of test sample into the syringe. Invert the syringe and eject to remove any air. Wipe any excess liquid from the needle. Obtain a mass to +/- 0.1 mg (W1). See Table 1.
13.1.1 A 1 mL gas tight syringe is suggested for single coulometric injections. If replicates of the same sample are required, a larger syringe with suitable volume may be used. The sample volume should occupy at least 25 % of the syringe volume. For increased precision, it is not recommended to inject less than 0.25 mL of sample into the coulometric cell.
13.2 With the analyzer stabilized, carefully insert the needle of the sample syringe through the septum and slightly below the level of solution in the titration chamber. Inject the sample carefully into the titration solution and begin titration per manufacturer directions. Withdraw the syringe needle and weigh to the nearest +/- 0.1 mg (W2) to determine the exact sample mass. Allow the titration to proceed until the end-point is indicated.
13.2.1 After numerous analyses, the level of solvent accumulated in the titration chamber may have to be reduced. This can be accomplished with a syringe of capacity of 20 mL or by partially draining the solution of the titration chamber. Discard the solution and replace with fresh anolyte solution if a stable reading cannot be obtained.
13.2.2 When a stable reading cannot be obtained, replace the reagents and follow the manufacturer procedure to condition the reagents.
13.3 End Point Detection:
13.3.1 During coulometric titrations, iodine is generated electrochemically by anodic oxidation of iodide to iodine. There is a quantitative relationship between the amount of electric current passed through the generator electrode and the amount of iodine generated. Iodine will be consumed as long as water is present.
13.3.2 End point is detected automatically and the water content is calculated based on the sample weight entered. During the titration a small constant polarization current is applied to the double platinum electrode and the voltage required to maintain this current is measured. When water is present in the titration vessel, the voltage required is high. Once there is a slight excess of iodine, the voltage required is reduced. This large change in voltage indicates the titration end point.
13.4 Record the micrograms of water determined for the sample titration.
14. Calculation
14.1 The water content is manually calculated in percent by mass using Eq 1 or percent by volume using Eq 2. Most instruments are equipped to provide a calculated result based upon the measured sample size.
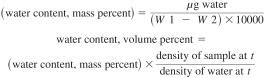
where:
t = test temperature,
W1 = mass of sample and syringe before injection, g, and
W2 = mass of sample and syringe after injection, g.
15. Report
15.1 Report the percentage of water to the nearest 0.01 % by mass. Alternatively, report the percentage of water to the nearest 0.01 % by volume.
15.2 Report the water concentration in one of the defined units as obtained by Test Method D7923, Procedure A.
16. Precision and Bias
16.1 The statistical precision of this procedure, as determined by statistical examination of the 2015 interlaboratory test results, obtained from 12 laboratories on 12 samples, is as follows:
16.1.1 Repeatability - The difference between successive results obtained by the same operator with the same apparatus under constant operating conditions on identical test material, would in the long run, in the normal and correct operation of the test method, exceed the following values in one case in twenty.
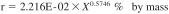
where:
X = the calculated result for percentage of water expressed as percent by mass.
16.1.2 Reproducibility - The difference between two single and independent results, obtained by different operators working in different laboratories on identical material, would be in the long run, in the normal and correct operation of the test method, exceed the following values only in one case in twenty.
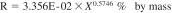
where:
X = the calculated result for percentage of water expressed as percent by mass.
NOTE 3 - The data in Table 2 shows repeatabilities and reproducibilities for water values obtained using the formulas given in 16.1.1 and 16.1.2.
16.1.3 The precision statement was determined through statistical examination of 11 materials with blind duplicates from 12 laboratories. The materials included one sample of anhydrous ethanol, six ethanol blends ranging from a nominal 5 % to 85 % ethanol, three samples of denatured fuel ethanol, and one sample of gasoline. Water contents of the samples ranged from 0.002 % by mass to 1.63 % by mass by Procedure A.
16.2 Bias - This test method has no bias since the coulometric test method can be defined only in terms of this test method.

