ASTM D7169 Standard Test Method for Boiling Point Distribution of Samples with Residues Such as Crude Oils and Atmospheric and Vacuum Residues by High Temperature Gas Chromatography
13. Verification of System Performance
13.1 Column Resolution - Prepare the gas chromatograph for injection of the retention time calibration mixture prepared in 8.4. Inject 0.1 to 0.2 µL of this sample. Determine the column resolution as follows:
R = 2(t2 - t1)/(1.699)(W2 + W1)
where:
R = resolution,
t2 = retention time (s) for the n-C50 paraffin,
t1 = retention time (s) for the n-C52 paraffin,
W1 = peak width (s) at half height of the n-C50 peak, and
W2 = peak width (s) at half height for the n-C52.
13.1.1 Ensure that the resolution, R, is between 1.8 to 4.0.
13.2 Skewness Test for Column Overloading - Select a component between C12-C24 of the previous chromatogram or of the chromatogram of the retention time calibration mixture prepared in 8.4. For the component selected, determine the skewness as follows. The skewness, s, is calculated by Eq 2: ILS participants reported skewness of 0.8 to 2.0 for peaks C7 to C100.
s = (a + b)/2a
where:
s = skewness of the peak,
a = left time segment measured at 10 % of the peak height and that intersects the perpendicular from the apex of the peak to the retention time axis, and
b = right time segment measured at 10 % of the peak height and that intersects the perpendicular from the peak apex to the retention time axis. Ensure that the skewness is between 0.8 to 1.2. Data acquisition systems can calculate this parameter.
13.3 Determination of Detector Relative Response Factors - Prepare the gas chromatograph for the injection of the detector test mixture prepared in 8.5. Inject 0.1 to 0.2 µL of this sample. Calculate the relative response factor, Fi, of each paraffin relative to eicosane as follows:
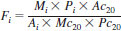
where:
Mi = mass of the paraffin in mg,
Mc20 = mass of the eicosane in mg,
Ai = peak area of the paraffin,
Ac20 = peak area of the eicosane,
Pi = % purity of the paraffin as recorded in 8.5.3, and
Pc20 = % purity of eicosane.
13.3.1 The relative response factor, Fi, should have a value of between 0.95 to 1.05. Failure to achieve this range may be due to inlet problems, lack of constant flow, or partial blockage of the flame tip orifice, or a combination thereof.
14. Analytical Sequence
14.1 Set up a sequence of the samples to be analyzed. The sequence will contain the order of the samples to be injected into the column. This schedule should be designed to achieve maximum reproducibility. A suggested order of the samples to be analyzed is described in 14.2-14.6. If time constraints require a shorter sequence, the user shall ensure that there is no carryover between samples and sample types.
14.2 Blank Run - At the beginning of each sequence, after any column maintenance is performed, make a blank run. It may take more than 2 blanks to show a stable plateau with no indication of residual elution. A blank run constitutes an identical solvent injection having the same volume as the sample injection. An acceptable blank run should show a stable plateau at the highest temperature of the oven (see 15.3). Furthermore, it should not show any indication of carryover or residual sample elution. It should also not contain any ghost peaks. A typical blank sample run is shown in Fig. A1.1. Several blanks may be necessary after column installation or after an idle period of the gas chromatograph.
14.3 Retention Time Calibration Mixture - Insert the retention time calibration mixture vial prepared in 8.4 into the auto sampler for injection. Insert the vial again at the end of analysis in order to ascertain the stability of the column. A typical chromatogram of the retention time calibration mixture is shown in Fig. A1.2. The insert in the Fig. A1.2 shows the best separation possible for the C5, CS2, C6, and C7 and shows good peak shape for the C6 and C7 hydrocarbons. Identify all carbons up to C100.
14.4 Response Factor Standard - Insert the vial containing Reference Oil 5010 prepared in 8.5, which is used as a response factor standard. Inject this standard in duplicate. A typical chromatogram of the reference oil analyzed at an initial oven temperature of -20°C is shown in Fig. A1.3. Verify that the response factor calculated by Eq 4 does not vary by more than 2%.
14.5 Sample Analysis - Insert the sample vials prepared in 10.3. Inject samples. It is suggested to inject samples in duplicate and to observe, by overlaying the chromatograms, that the retention times of the components do not vary by more than 3 s and that signal amplitudes are similar.
14.6 Additional Blank Runs - Insert a vial containing CS2 in order to obtain a second blank run. Carry out a blank run after each sample injection, and verify the absence of carryover from the previous samples.
15. Verification of Acquired Data
15.1 Inspect all chromatograms by loading the data files in the data acquisition system. Observe that the signal magnitude for each sample injected is approximately the same as that for the retention time calibration mixture and the Reference Oil 5010 chromatograms.
15.2 Verification of the Retention Time Calibration Mixture Chromatogram - Inspect the chromatograms acquired during a sequence run. Verify that, in duplicate injections, the retention time of each of the paraffins does not differ by more than 3 s. Do not use a chromatogram where the peaks do not meet the criteria of skewness as defined in 13.2. Inspect the chromatogram for the components C5-C7 and the solvent peak as shown in the insert of Fig. A1.2. The peaks should not present peak splitting nor peak tailing.
15.3 Sample Chromatograms - Inspect the sample chromatograms and verify that the chromatograms can be overlaid to a duplicate chromatogram and show that the profile is reproducible. Check that the retention times in duplicate runs do not differ from each other by more than 3 s. Fig. A1.4 shows a chromatogram of a 30°API crude oil where the solvent peak is not resolved from the sample components. Fig. A1.5 shows a typical chromatogram of an atmospheric residue where the solvent peak is resolved from the sample components.
15.3.1 A QC material should be analyzed with every sequence. The QC sample should have the same matrix as the samples analyzed, and a line should be inserted in the sequence after every tenth sample.
15.4 Baseline or Blank Runs - Inspect, in the data system, the chromatograms of the blank solvent injections to verify that the blank signal obtained does not differ substantially from that obtained during the sample analysis. Check that the baseline exhibits a gradual rise up to the isothermal section of the chromatogram and ensure that there is a gradual transition back to the plateau of the baseline. Disregard any baseline that shows material eluting near the highest temperature of the column. Also disregard any baseline that shows ghost peaks. Overlay the baseline signal with the sample signal as shown in Fig. A1.6. Use only those sample signals that asymptotically approach the baseline signals. Reject any sample run where the baseline signal at the end of the run exceeds in value the sample run. Reject any sample run at which at the end of the run the signal exceeds the baseline signal by 10 %.
15.4.1 Determine the Quenching Interval - Select the time that the solvent peak starts to elute. Determine when the solvent peak has eluted. Note the times of this interval in minutes. An expanded time scale chromatogram of the solvent peak is shown in Fig. A1.7.
15.4.2 Determine the Magnitude of Solvent Response - Using the data system, overlay the solvent chromatograms and verify that the profiles are similar. Verify that the total areas do not differ by more than 3 % from each other.
15.5 External Standard Response Factor Chromatogram - Inspect the external standard chromatogram obtained from the injection of Reference Oil 5010. Verify that the boiling point distribution is within the consensus values as indicated in Test Method D6352. Typical boiling point distribution values for Reference Oil 5010, obtained with this test method, are shown in Table 2. Correct any chromatography errors if the consensus values are not obtained (see 16.1.7).

