8. Sampling
8.1 Container Sampling - Samples shall be taken as described in Practice D4057 for instructions on manual sampling into open container.
8.2 The sample and a 2-mL vial must be cooled at 4°C. Part of the sample is transferred to the vial up to 80 % of its volume, and aluminum cap with septum is crimped.
9. Preparation of Apparatus
9.1 Installation - Install and condition column in accordance with the supplier's instruction.
9.2 Operating Conditions - Two sets of operating conditions are proposed in Table 1, the first with an initial column temperature above the ambient temperature, the second with a sub-ambient column temperature profile. Adjust the operating conditions of the gas chromatograph to conform to the first or second method.
9.3 Carrier Gas Pressure - Set a correct carrier gas pressure using the system performance mixture such that the retention time of n-Heptane, n-Octane and n-Dodecane are between the values given in Table 2.
10. System Performance Evaluation
10.1 Evaluation of the column and linearity of the split injection are carried out with a system performance mixture defined in 7.12 and with the column temperature conditions defined in the following table.

10.2 Column Evaluation - To perform the required separation, the column must meet three criteria of separation: efficiency, resolution, and polarity.
10.2.1 Efficiency - The number of theoretical plates is calculated with the normal octane peak using Eq 1:
n = 5.545 (Rt/W0.5)2
where:
n = number of theoretical plates,
Rt = retention time of normal octane, and
W0.5 = mid-height peak width of normal octane in the same unit as retention time.
10.2.1.1 The number of theoretical plates must be greater than 200 000.
10.2.2 Resolution - Resolution is determined between the peaks of 2-methylheptane and 4-methylheptane using Eq 2:
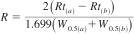
where:
Rt(a) = retention time of 4-methylheptane,
Rt(b) = retention time of 2-methylheptane,
W0.5(a) = mid-height peak width of 4-methylheptane in the same unit as retention time, and
W0.5(b) = mid-height peak width of 2-methylheptane in the same unit as retention time.
10.2.2.1 The resolution must be equal to 4 or greater than 1.20.
10.2.3 Polarity - Polarity is defined by the McReynolds constant of toluene, using Eq 3:
Rntol = Kiana - Kisqualane
where:
Kisqualane = toluene Kovats index on Squalane at 35°C = 742.6, and
Kiana = toluene Kovats index on the analytical column at 35°C.
10.2.3.1 Toluene Kovats index is calculated using Eq 4:
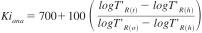
where:
T'R(t) = adjusted retention time for toluene,
T'R(h) = adjusted retention time for n -heptane, and
T'R(o) = adjusted retention time for n-octane.
10.2.3.2 Adjusted retention time of a peak is determined by subtracting the retention time of an unretained compound (air or methane) from the retention time of the peak. The McReynolds constant must be less than 10.
10.2.4 Base Line Stability - Base line stability is calculated with the difference between area slices at the beginning and at the end of analysis, divided by the maximum area slice of N-octane obtained with the system performance mixture.
10.2.4.1 Measurement of the Stability - Carry out one temperature programming defined in 10.1 without injecting any sample. Subtract the area slices at the start of the analysis with those corresponding to 120 min (average of three slices).
10.2.4.2 Stability Standardization - Standardization is carried out using the system performance mixture defined in 7.12 with the column temperature conditions defined in 10.1. The value obtained in 10.2.4.1 is divided by the maximum area slice of N-octane and multiplied by 100. The value obtained must be less than 2 %. If this is not the case, check for possible leaks, or recondition the column according to the manufacturer's recommendations.
10.3 Evaluation of the Linearity of the Split Injector - Evaluation is carried out using the system performance mixture defined in 7.12 with the column temperature conditions defined in 10.1. The % (m/m) of each compound is determined from the corrected area % using the response factors for each compound given in Table A1.1 or Table A1.2. The relative percent error is determined from the known mixture concentrations according to Eq 5:
Relative% error = 100 (calculated concentration - known concentration)/known concentration
10.3.1 The relative error must not exceed 3 %.
11. Response Factor
11.1 Theoretical response factors are used for correction of the detector response of hydrocarbons. The response factor for each compound is relative to that of benzene taken equal to unity and is listed in Tables 1 and 2. For peaks corresponding to the co-elution of compounds with benzene, toluene, and oxygenates, the response factor is the one of the co-eluted compound of% (m/m). Co-eluted compounds are footnoted in Tables A1.1 and A1.2.

