12. Procedure
12.1 Set up the closed respirometer (see the examples described in 6.1, manufacturer's manual, ISO 9408:1999, or OECD 301F:1992). For each blank, test material, and reference material being tested, prepare the following (based on 1-L test system volume):
12.1.1 Prepare the test medium by adding 850 mL of water to each of the respirometer flasks. To each of the respirometer flasks, add 10 mL of the phosphate buffer solution, 1mL of magnesium sulfate solution, 1 mL of ferric chloride solution, and 1 mL of calcium chloride solution.
12.2 Addition ofInoculum:
12.2.1 Addition of Non-adapted Homogenized Mixed Liquor - Add sufficient volume of the homogenized mixed liquor inoculum (8.1.1.2) to each of the respirometer flasks to give 30 mg/L of suspended solids.
12.2.2 Addition of non-adapted activated sludge supernatant (8.1.1.3); secondary effluent (8.1.2.1); surface water (8.1.3.1); soil (8.1.4.3); or composite (8.1.5). Add 10 mL of the selected inoculum to each of the respirometer flasks.
12.2.3 Addition of Adapted Inoculum - Add 100 mL of pre-adapted inoculum (8.3.4) to each of the respirometer flasks (see Test Method D1293).
12.3 Measure the pH in each flask and adjust to pH 7.4 +/- 0.2, if necessary, with dilute HCl or NaOH before adding the test material or reference material.
12.4 Addition ofTest Material or Reference Material:
12.4.1 The concentration of the test material or reference material in the test medium shall be approximately 50 to 100 mg/L, providing at least 50 mg ThO2 in the test medium, but no more than 200 mg ThO2 . Calculate the ThO2 to ensure this is within the range specified. Decrease or increase the amount of material necessary to achieve a ThO2 within the specified range.
12.4.2 Add the test material or reference material gravimetrically to the replicate respirometer flasks. If, in order to accomplish this, the material is weighed into or onto a small object, then both the material and object shall be added to the flask.
NOTE 2 - An example of a small object might be a glass fiber filter. The test or reference material is added to the respective shake flasks as a measured weight adsorbed to the surface of the filter. This enables an accurate weight to be dosed into each flask and increases the surface area of the material. A blank glass fiber filter should also be added to each blank flask.
12.4.3 Sonication of the test material or reference material in 5 mL of water while still in or on a small object is allowed as a means of obtaining a better dispersion of insoluble materials in the test medium. If sonication is performed, the object shall also be added to the flask. In addition, ifsonication is performed on the test material, the reference material shall also be sonicated in an identical manner prior to its addition to the test medium.
12.4.4 Along with the flasks containing test material or reference material, additional replicate flasks shall contain the test medium and the inoculum with no other additions. These flasks shall be blanks.
12.4.5 Add sufficient volume of water to achieve a final volume of 1000 mL in each flask.
12.4.6 Add sufficient alkaline solution (10 M NaOH or KOH), or other suitable absorbent to the CO2 -absorber compartments ofthe respirometer. These solutions may be prepared in the laboratory or obtained commercially. A 10 M NaOH solution is prepared by cautiously dissolving 400 g NaOH in distilled water to a final volume of 1 L. A 10 M KOH is prepared by dissolving 658.8 g KOH in distilled water to a final volume of 1 L. Filter each solution to free it of solid material, confirm molarity by titration with standard acid, and store under nitrogen, sealed to prevent absorption of CO2 from the air.
12.4.7 Seal the flasks and, in the case of automatic respirometers, perform any procedures as specified in the manufacturer's manual to initiate oxygen consumption measurements, and start stirring the contents of each flask.
12.4.8 Incubation shall take place in the dark or diffused light, in an enclosure that is maintained at a constant temperature (within at least 1°C) between 20 and 24°C. Record the test temperature throughout the test.
12.4.9 Take necessary readings on the manometers (if manual) and verify that the oxygen consumption data is being recorded properly (automatic respirometer).
12.4.10 Stop the test after the specified period, (usually 28 days), or earlier if a plateau of oxygen consumption has been attained. The test may be extended as long as the systems' integrity is maintained and the inoculum in the blank system is viable. The duration of the test will be dependent on the length of time required for the rate of test material biodegradation to achieve a plateau.
12.4.11 Measure the final pH value of the flask contents at the end of the test.
13. Calculation and Expression of Results
13.1 Calculation:
13.1.1 The ThO2 of the material Cc Hh Clcl Nn Nana Oo Pp Ss, of empirical weight Mr, can be calculated according to:
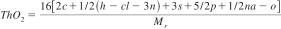
13.1.2 This calculation implies that C is mineralized to CO2, H to H2O, P to P2O5, and Na to Na2O. The Cl is eliminated as hydrogen chloride and nitrogen as ammonia. Sulfur is assumed to be oxidized to the S(+6) oxidation state.
13.1.2.1 Example of the calculation of the theoretical oxygen demand: glucose (C6H12O6), Mr = 180 g/mol.
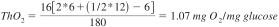
13.1.2.2 Empirical molecular weights of salts other than those of the alkali metals are calculated on the assumption that these salts have been hydrolyzed.
13.1.3 Example of the calculation of the theoretical oxygen demand: sodium n-dodecylbenzenesulfonate (C18H29SO3Na), Mr = 348 g/mol
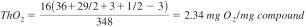
13.2 Calculate the oxygen consumption values for each flask at selected time intervals, from the reading obtained, using the method given by the manufacturer for the appropriate type of respirometer. Calculate the oxygen demand in milligrams per litre of the test material as the difference between oxygen consumption in the test flask and the mean oxygen consumption of the blank flasks. Divide this difference by the concentration of the test material to give the net oxygen consumption expressed as specific BOD in milligrams of O2 per milligram of test material.
13.2.1 Specific BOD at selected time intervals
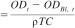
where:
ODt = oxygen consumption of the test material solution at time, t, mg/L,
ODBl, t = mean oxygen consumption of the blanks at time, t, mg/L, and
ρTC = mass concentration of the test material, mg/L.
13.2.2 The degradation is defined as the ratio of the specific biochemical oxygen demand to the theoretical oxygen demand (ThO2). Determine the percentage degradation (Dt) for each test flask, using the following equation:
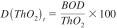
where:
D(ThO2)t = percentage biodegradation of ThO2 at time t.
13.2.3 These calculations may be automatically generated by specific commercial respirometers or by specific software data analysis systems interfaced to the respirometer.
13.3 Plot the percentage degradation, Dt for each flask against time to obtain the degradation curve (see example in 4.5). Draw an average curve if comparable results in the parallel test flasks are obtained.
13.4 If sufficient data are available, indicate clearly on the curve the lag time, the maximum level of degradation, and the degradation time.



