ASTM D664 for acid number of petroleum products by potentiometric titration
11. Procedure for Acid Number and Strong Acid Number
11.1 Into a 250-mL beaker or a suitable titration vessel, introduce a weighed quantity of sample as recommended in Table 1 (see Note 13) and add 125 mL of titration solvent (see Note 14). Prepare the electrodes as directed in 8.2. Place the beaker or titration vessel on the titration stand and adjust its position so that the electrodes are about half immersed. Start the stirrer, and stir throughout the determination at a rate sufficient to produce vigorous agitation without spattering and without stirring air into the solution.
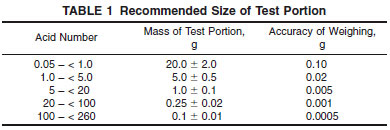
NOTE 13 - If it suspected that the recommended sample size will foul the electrodes, a smaller sample size can be taken. Results using smaller sample size may not be equivalent to results obtained with the recommended sample size. The precision statement does not include results when using a smaller sample size.
NOTE 14 - A titration solvent that contains chloroform (Warning - May be fatal if swallowed. Harmful if inhaled. May produce toxic vapors if burned) can be used in place of toluene to completely dissolve certain heavy residues of asphaltic materials. Results using chloroform may not be equivalent to results obtained using toluene. The precision statement does not include results when using chloroform.
11.2 Select the right burette, fill with the 0.1-mol/L alcoholic KOH solution, and place the burette in position on the titration assembly, ensuring that the tip is immersed about 25 mm in titration vessel liquid. Record the initial burette and meter (cell potential) readings.
11.3 Manual Titration Method:
11.3.1 Add suitable small portions of 0.1-mol/L alcoholic KOH solution and wait until a constant potential has been established, record the burette and meter readings.
11.3.2 At the start of the titration and in any subsequent regions (inflections) where 0.1 mL of the 0.1-mol/L KOH solution consistently produces a total change of more than 30 mV in the cell potential, add 0.05-mL portions.
11.3.3 In the intermediate regions (plateau) where 0.1 mL of 0.1-mol/L alcoholic KOH changes the cell potential less than 30 mV, add larger portions sufficient to produce a total potential change approximately equal to, but not greater than 30 mV.
11.3.4 Titrate in this manner until the potential changes less than 5 mV/0.1 mL of KOH and the cell potential indicates that the solution is more basic than the aqueous basic buffer.
11.3.5 Remove the titration solution, rinse the electrodes and burette tip with the titration solvent, then with propan-2-ol and finally with reagent grade water. Immerse the electrodes in water for at least 5 min before starting another titration to restore the aqueous gel layer of the glass electrode. After 5 min in the water, rinse the electrodes with propan-2-ol then the titration solvent before proceeding to the next titration. If the electrodes are found to be dirty and contaminated, proceed as in 8.1. Store electrodes according to 8.3.3.
11.4 Automatic Titration Method:
11.4.1 Adjust the apparatus in accordance with the manufacturer's instructions to provide a dynamic mode of titrant addition.
11.4.2 Verify that the instrument will determine the amount of strong acid when the initial mV of the test sample, relative to the mV reading of the aqueous acidic buffer, indicates the presence of such acids. Record the volume of KOH added to reach the mV of the pH 4 aqueous buffer. This value is used to calculate the strong acid number. Proceed with the automatic titration and record potentiometric curves or derivative curves as the case may be.
11.4.3 Titrate with the 0.1-mol/L alcoholic KOH solution. The apparatus shall be adjusted or programmed such that, when an inflection point, suitable for use in the calculation is approached, the rate of addition of titrant and volume of titrant added are based on the change in slope of the titration curve. The titrant shall be added in increments of a suitable size to achieve a potential difference of 5 to 15 mV per increment. Increment volume shall vary between 0.05 and 0.5 mL. The next increment shall be added if the signal does not change more than 10 mV in 10 s. The maximum waiting time in between increments shall not exceed 60 s.
11.4.4 The titration can be terminated when the signal reaches the pH 11 buffer potential past 200 mV. An equivalence point is recognizable if the first derivative of the titration curve produces a maximum, which is significantly higher than the noise produced by electrostatic effects. See also 12.1.1.
11.4.5 On completion of the titration, rinse the electrodes and burette tip with the titration solvent, then with propan-2-ol, and finally with reagent grade water. Immerse the electrodes in water for at least 5 min before starting another titration to restore the aqueous gel layer of the glass electrode. Rinse the electrodes with propan-2-ol and finally with the titration solvent prior to running the next sample. If electrodes are found dirty and contaminated, proceed as in 8.1. Store electrodes according to 8.3.3.
NOTE 15 - When acid numbers about or below 0.1 are expected, better precision can be obtained by modifying the method in one or more ways, such as by substituting a 0.01 or 0.05 M alcoholic KOH solution; increasing the sample size above 20 g; or switching f rom a manual operated burette (that is, graduated in 0.05 mL divisions) to an automated burette that can dispense smaller increments of the KOH solution, if samples are being analyzed by manual titration.
11.5 Blanks:
11.5.1 For each set of samples and for every new batch of titration solvent, perform a blank titration of 125 mL of the solvent. For manual titration, add 0.1-mol/L alcoholic KOH solution in 0.01 to 0.05-mL increments, waiting between each addition until a constant cell potential is reached. Record the meter and readings when the former becomes constant after each increment. For automatic titration, use the same mode of titration as for the determination of the acidic property of the sample but use smaller increments of titrant addition, 0.01 to 0.05-mL. Recheck the blank periodically based on the sample load.
11.5.2 When strong acids are present and a strong acid number is to be determined, perform a blank titration of 125 mL of the titration solvent, adding 0.1 mol/L alcoholic HCl solution in 0.01 to 0.05-mL increments in a manner comparable to that specified in 11.5.1.

