ASTM D6445 Standard Test Method for Sulfur in Gasoline by Energy-Dispersive X-ray Fluorescence Spectrometry
8. Sampling and Specimen Preparation
8.1 Take samples in accordance with the instructions in Practice D4057 or D4177 where appropriate. Thoroughly mix and analyze samples immediately after pouring into a sample cell. Inspect the sample for any air bubbles or sediment. Allow air bubbles to escape or resample if necessary.
NOTE 4 - The measured sulfur concentration may vary with the time that the sample/standard contacts the film covering the sample cell. By consistently minimizing the length of time the film comes into contact with the sample or standards, possible variations can be reduced.
8.2 If using reusable sample cells, clean and dry cells before use. Do not reuse disposable sample cells. Replacement of the X-ray film of a reused sample cell is essential for the measurement of each sample. Avoid touching the inside of the sample cell or portion of the window film in the cell or in the instrument window that is exposed to X rays. Oil from fingerprints can affect the reading when analyzing for low levels of sulfur. Wrinkles in the film will affect the intensity of sulfur X rays transmitted. Therefore, it is essential that the film be taut and clean to ensure reliable results. The analyzer will need recalibration if the type or thickness of the window material is changed.
8.3 Impurities or thickness variations, which may affect the measurement of low levels of sulfur, have been found in window materials films and may vary from lot to lot. Therefore, check the calibration after starting each new package of film.
9. Preparation of Apparatus
9.1 Set up the apparatus in accordance with the manufacturer's instructions. Whenever possible the instrument should remain energized to maintain optimum stability.
10. Calibration and Standardization
10.1 Preparation of Calibration Standards:
10.1.1 Prepare diluent by blending 20 % toluene and 80 % isooctane by volume.
10.1.2 Use either di-n-butyl sulfide or a blend of thiophene/2-methylthiophene as a source of sulfur in the primary standards. If using di-n-butyl sulfide as the source of sulfur, proceed to 10.1.3.
10.1.2.1 To prepare the thiophene/2-methylthiophene (TM) blend, mix 9.90 g thiophene with 9.55 g 2-methylthiophene. Weigh the materials into a tared volumetric flask and record the mass to four significant digits. Calculate the exact sulfur content of the stock sulfur solution to the nearest mg/kg. Mix thoroughly (a polytetrafluoroethylene (PTFE)-coated magnetic stirrer is suitable) at room temperature.
10.1.3 Make primary standards independently at 100 and 2000 mg/kg sulfur and not by serial dilutions from a single concentrate. Refer to 10.1.3.1 if using di-n-butyl sulfide and 10.1.3.2 if using the thiophene/2-methylthiophene blend for the source of sulfur.
10.1.3.1 Weigh the diluent and the di-n-butyl sulfide (DBS) into a tared volumetric flask, using the indicated mass in Table 1 (but record the mass to four significant digits). Mix thoroughly (a PTFE-coated magnetic stirrer is suitable) at room temperature.
10.1.3.2 Weigh the diluent and the thiophene/2-methylthiophene (TM) blend into a tared volumetric flask using the indicated mass in Table 2 (but record the mass to four significant digits). Mix thoroughly (a PTFE-coated magnetic stirrer is advisable) at room temperature.
10.1.3.3 If the isooctane/toluene diluent being used for the preparation of standards contains sulfur, incorporate this value into the calculated sulfur content of the prepared standards (consult your supplier for a certified sulfur concentration or test the isooctane/toluene using Test Method D3120 or any other equivalent low level sulfur analyzing method).
10.1.3.4 It is important that the actual mass is known and thus the actual concentration of the prepared standards is calculated and entered into the instrument for calibration purposes. Calculate the exact sulfur content in each of the prepared standards to the nearest 1 mg/kg. Calculate the concentration of sulfur in the primary standard using the following equations. Use Eq 1 if using di-n-butyl sulfide and Eq 2 if using the thiophene/2-methylthiophene blend as a source of sulfur:

where:
S = mg/kg sulfur of the primary standards,
DBS = if using the di-n-butyl sulfide, this is the actual mass in grams of di-n-butyl sulfide used,
TM = if using the thiophene/2-methylthiophene blend, this is the actual mass of the sulfur blend in grams,
SDBS = if using di-n-butyl sulfide, this is the mass % sulfur of the di-n-butyl sulfide, typically 21.91 %. For example, 21.91 % would be expressed as 21.91 in the formula (see Note 3),
STM = if using the thiophene/2-methylthiophene blend, this is the mass % sulfur in the this blend, typically 34.9 mass %. For example, 34.9 % would be expressed as 34.9 in the formula (see Note 3),
Diluent = actual mass of isooctane/toluene diluent (g), and
SDiluent = mass % sulfur in the isooctane/toluene blend. For example, 0.0001 % would be expressed as 0.0001 in the formula.
10.1.4 Prepare calibration standards with the nominal concentration ranges identified in Table 3 for the two ranges by diluting the appropriate primary standard with diluent. Adjust masses as needed if preparing more or less than 100 g of standard solutions.
10.1.4.1 Calculate the exact sulfur content in each of the calibration standards to the nearest mg/kg. Calculate the concentration of sulfur using the following equation:
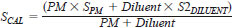
where:
SCAL = mg/kg sulfur of the calibration standards,
PM = this is the actual mass in grams of the primary standard used,
SPM = this is the mg/kg sulfur of the primary standard. For example, 100 mg/kg would be expressed as 100 in the formula,
Diluent = actual mass in grams of the isooctane/toluene diluent, and
S2Diluent = mg/kg sulfur in the isooctane/toluene blend. For example, 0.5 mg/kg would be expressed as 0.5 in the formula.
10.1.5 Alternatively, nationally traceable certified standards, such as National Institute of Science and Technology (NIST), prepared as described above or composed of the matrix to be analyzed can be used.
10.2 Certified Calibration Standards - Calibration standards, which are certified by an organization in accordance with a protocol that is technically equivalent to that used by NIST for certification of standard reference materials organization, may be used when they cover the nominal concentrations in Table 2 and are applicable to the sample of interest.
10.3 Quality Control (QC) Standards - Use several additional standards (QC standards) that were not used in generating the calibration curve to check the validity of the calibration. QC standards may be independently prepared as per 10.1 or certified standards as per 10.2. The concentration and matrices of the QC standards shall be near the expected concentration of the samples being analyzed.
10.4 Storage of Standards and QC Standards: Store all standards in glass bottles, either dark or wrapped in opaque material, closed with glass stoppers, inert plastic lined screw caps, or other equally inert, impermeable closures, in a cool, dark place until required. As soon as any sediment or change of concentration or stratification is observed, discard the standard.

