ASTM D6378 Standard Test Method for Determination of Vapor Pressure (VPX) of Petroleum Products, Hydrocarbons, and Hydrocarbon-Oxygenate Mixtures (Triple Expansion Method)
8. Sampling and Sample Introduction
8.1 General Requirements:
8.1.1 The extreme sensitivity of vapor pressure measurements to losses through evaporation and the resulting changes in composition is such as to require the utmost precaution and the most meticulous care in the drawing and handling of samples.
8.1.2 Obtain a sample and test specimen in accordance with Practice D4057, D4177, D5842, or D5854 when appropriate, except do not use the Sampling by Water Displacement section for fuels containing oxygenates. Use a container not less than 300 mL and not more than 1 L in size filled at least 70 % with sample.
NOTE 7 - The present precision statement was derived using samples in 1 L (1 qt) containers. However, samples in containers of other sizes as prescribed in Practice D4057 can be used, with the same filling requirement, but the precision can be affected.
8.1.3 Perform the VPX determination, including the rinsing (see 9.4), on the first test specimen withdrawn from a sample container. Do not use the remaining sample in the container for a second VPX determination. If a second determination is necessary, obtain a new sample.
8.1.4 Protect samples from excessive temperatures prior to testing. This can be accomplished by storage in an appropriate ice bath or refrigerator.
8.1.5 Do not test samples stored in leaky containers. Discard and obtain a new sample if leaks are detected.
8.2 Sampling Handling Temperature - Cool the sample container and contents in a ice bath or refrigerator to the 0 to 1°C (32 to 34°F) range prior to opening the sample container. Allow sufficient time to reach this temperature. Verify the sample temperature by direct measurement of the temperature of a similar liquid in a similar container placed in the cooling bath or refrigerator at the same time as the sample.
NOTE 8 - Cooling of the sample may be omitted if provisions are provided that avoid loss of high volatile components during the sample introduction. Sample introduction with a pressurized sample container is a useful procedure. If a statement for hazy sample is required, cooling to the required temperature is necessary.
8.3 Verification of Sample Container Filling - With the sample at a temperature below 10°C, take the container from the cooling bath and wipe dry with absorbent material. If the sample is contained in a transparent container, verify that the container is at least 70 % full by suitable means, such as by using a marked ruler or by comparing it to a like container that has the 70 % levels clearly marked. If the container is not transparent, unseal it and, using a suitable gage, confirm that the sample volume equals at least 70 % of the container capacity.
8.3.1 Discard the sample if the container is filled to less than 70 %, by volume, of the container capacity.
8.3.2 Reseal the container, if opened, and place it back in the cooling bath or refrigerator.
8.4 Verification of Single Phase Sample - After drawing the test specimens and transferring them into the instrument for analysis, check the remaining sample for phase separation. If the sample is contained in a transport container, this observation can be made prior to sample transfer. If the sample is contained in a non-transparent container, shake the sample thoroughly and immediately pour a portion of the remaining sample into a glass container and observe for evidence of phase separation. A hazy appearance is to be carefully distinguished from separation into distinct phases. If the sample separates into two distinct phases with a discernible common boundary, then discard the test and the sample. If the sample has a hazy appearance, but does not have two distinct phases, then phase separation has not occurred. The test is valid, but the precision and bias in Section 15 may not apply (see Section 14).
9. Preparation of Apparatus
9.1 Prepare the instrument for operation in accordance with the manufacturer's instructions.
9.2 Rinse the measuring chamber, if necessary, with a solvent. Methanol has a low vapor pressure and can be used successfully. Rinsing is performed by drawing the solvent into the chamber by the piston and expelling the solvent into the waste container.
9.3 To avoid contamination of the test specimen with the previous sample or the solvent, rinse the measuring chamber a minimum of three times with the sample to be tested. Fill the measuring chamber with sample to at least half the total volume of the chamber for each rinse. This rinsing procedure shall always be carried out immediately before the measuring procedure (see 12.2).
9.4 If a syringe is used for introduction of the test specimen, chill the syringe to the temperature of the sample (below 10°C) in an air or water bath before drawing in the specimen. Avoid water contamination of the syringe reservoir by suitably sealing the syringe during the cooling process.
10. Calibration
10.1 Pressure Transducer:
10.1.1 Check the calibration of the transducer on a monthly basis or when required as indicated from the quality control checks (see Section 11). The calibration of the transducer is checked using two reference points: zero pressure (<0.1 kPa) and the ambient barometric pressure.
10.1.2 Connect a McLeod gage or a calibrated electronic vacuum measuring device to the vacuum source in line with the measuring chamber. Apply vacuum to the measuring chamber. When the external gage registers a pressure less than 0.1 kPa (0.8 mm Hg), adjust the transducer control to zero or to the actual reading on the external gage as dictated by the instrument design or manufacturer’s instructions.
NOTE 9 - Refer to Annex A1 of Test Method D1160 for further details concerning the calibration of pressure sensors and McLeod gages.
10.1.3 Open the measuring chamber of the apparatus to atmospheric pressure and observe the corresponding pressure value of the transducer. Ensure that the apparatus is set to display the TPX and not a calculated or corrected value. Compare this pressure value with the pressure obtained from a mercury barometer, or equivalent, as the pressure reference standard. The pressure measuring device shall measure the local station pressure at the same elevation as the apparatus in the laboratory at the time of pressure comparison.
NOTE 10 - Caution: Many aneroid barometers, such as those used at weather stations and airports, are precorrected to give sea level readings, these shall not be used for calibration of the apparatus.
10.1.4 Repeat 10.1.2 and 10.1.3 until the zero and barometric pressures read correctly without further adjustments.
10.2 Temperature Sensor - Verify the calibration of the platinum resistance thermometer used to monitor the measuring chamber temperature at least every six months against a thermometer that is traceable to National Institute of Standards and Technology (NIST) or to national authorities in the country in which the equipment is used.
11. Quality Control Checks
11.1 Use a verification fluid of known volatility as an independent check against the instrument calibration each day the instrument is in use. For pure compounds, multiple test specimens may be taken from the same container over time. If the observed VPX differs from the reference value by more than 1.0 kPa (0.15 psi), then check the instrument calibration (see Section 10).
11.2 Some possible materials and their corresponding vapor pressures at 37.8°C and a vapor-liquid ratio of 4:1 (VP4) include:
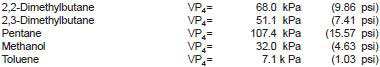
NOTE 11 - The value for 2,2-dimethylbutane in this list was derived from the 1991 interlaboratory cooperative test program and represents the VP4 values of the air saturated liquid. The other values were determined in limited cross check programs.
NOTE 12 - It is recommended that at least one type of verification fluid used in 11.1 have a vapor pressure representative of the fuel(s) regularly tested by the equipment. The vapor pressure measurement process (including operator technique) can be checked periodically by performing this test method on previously prepared samples from one batch of product, as per procedure described in 8.1.2. Samples should be stored in an environment suitable for long term storage without sample degradation. Analysis of result(s) from these quality control samples can be carried out using control chart techniques.
NOTE 13 - Caution: The use of single component verification materials, such as listed in 11.2, will only prove the calibration of the equipment. It will not check the accuracy of the entire test method, including sample handling, because losses due to evaporation will not decrease the sample vapor pressure as happens with losses of light ends in multi component mixtures.

