8. Sampling
8.1 Using a suitable H2S inert container of 250 to 500 mL, collect a representative sample by Practice D4057. Suitable containers can be made of borosilicate glass or aluminum. If the sample temperature is below 60°C then a high density polyethylene bottle can be used.
8.2 Fill the container completely to the top so that there is no headspace in the container. Cap immediately. (Warning - At no time should the container temperature be allowed to exceed the temperature of the sample at the time.)
8.3 Take the samples to the laboratory preferably within one to four hours, within 24 h maximum. Place the samples in refrigerated storage. Store samples until analysis time but not more than three days.
9. Preparation of Apparatus
9.1 Assemble the headspace sampling system as shown in Fig. 1.
9.2 Because of the chemical activity and adsorptive properties of H2S, it is highly desirable to connect the components of the test apparatus together using minimum lengths of aluminum or fluorocarbon sample lines. (Warning - To preclude the formation of mercaptide gels and to reduce problems associated with corrosion do not use brass or copper flow system parts.)
10. Calibration and Standardization
10.1 Filling Head Space Vial With Gas Calibration Standard:
10.1.1 Depending on the expected concentration use, a 1 µL/L (≤0.1µ g/g) in a 120-mL headspace vial, 10 µL/L (~1 µg/g) in a 60-mL headspace vial, or 100 µL/L (~10 µg/g) in a 30-mL headspace vial, H2S gas standard to calibrate the headspace sampling system.
NOTE 5 - Parts per million by volume units (µL/L), equivalent to micro moles per mole, are used because of the convenience in use of volume measurements rather than weight for a gas standard.
10.1.2 Insert a silicone/fluorocarbon septum, with the PTFE side pointing inwards, into the headspace vial, cover it with an aluminum seal, and crimp the aluminum seal with the hand crimper.
10.1.3 Insert the inlet needle attached to the H2S calibration cylinder (see Fig. 2) through the septum with the flow control set to zero. Also insert an open syringe needle into the septum as an outlet vent.
10.1.4 Set the pressure of the H2S calibration cylinder to 105 kPa, and open the flow control to purge the vial as follows:
(Warning - Because of the toxicity of H2S the vial should be in a hood during the purging operation.)
30-mL vial - purge for 3 min at a flow of 100 mL/min.
60-mL vial - purge for 6 min at a flow of 100 mL/min.
120-mL vial - purge for 10 min at a flow of 120 mL/min.
10.1.5 Turn the flow control to zero flow and remove the inlet needle, leaving the outlet vent in the vial.
10.1.6 Allow the vial to equilibrate to atmospheric pressure for 1.0 min, then remove the outlet vent.
10.1.7 Record the laboratory ambient temperature in °C and the laboratory barometric pressure in kPa.
10.1.8 Immediately after preparing a calibration standard obtain its analyzer response as shown in 10.2.1 - 10.2.7.
NOTE 6 - Alternative methods for preparing a calibration standard include: heating a solid to generate H2S and then using a permeation tube as discussed in Practice D3609 or using pure H2S and a movable piston as discussed in Test Method D4084.
10.2 Calibration of Analyzer:
10.2.1 Place the headspace vial in a 60°C oven for at least 5 min but for no more than 15 min. Install an 0.5-mL sample loop if using the 100 µL/L H2S standard, a 2.5 mL sample loop if using the 10 µL/L H2S standard, and a 10-mL sample loop if using the 1 µL/L H2S standard. With Valve 2 in the load position, see Fig. 1 and Valve 1 closed, evacuate the sample loop by opening Valve 3. When a vacuum of at least -70 kPag is achieved, then close Valve 3.
10.2.2 Immediately insert the sampling needle (Fig. 1) through the septum of the headspace vial containing the calibration gas mixture.
10.2.3 Open Valve 1 and let the sample fill the injection loop until a reading of 0 kPag is achieved on the pressure/vacuum gauge. Close Valve 1.
10.2.4 Place Valve 2 in the Inject position (see Fig. 1) for 10 s to allow the carrier gas to sweep the sample to the H2S analyzer. Return Valve 2 to the load position. Remove sampling needle from the vial. Record reading from the analyzer as reading A 1 .
10.2.5 After the injection step, insert needle connected to a line containing nitrogen at atmospheric pressure into the septum to reestablish atmospheric pressure within the vial.
10.2.6 Repeat steps 10.2.1 - 10.2.5 with the same headspace vial but record the reading from the analyzer as reading A2.
10.2.7 Repeat steps 10.2.1 - 10.2.5 with the same headspace vial but record the reading from the analyzer as reading A3. Two injections are sufficient for the required calculations, but three are required to verify the correctness of the calibration procedure.
10.2.8 Regress the natural log (ln) of readings A1, A2, and A3 against the number of injections one, two, and three. A linear correlation (correlation coefficient R2 > 0.95) must exist to insure the correctness of the calibration procedure. If R2 is not greater than 0.95, then repeat the calibration.
10.3 Analyzer Response Factor:
10.3.1 Calculate the total area difference (or total height difference) using Eq 1:
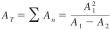
where:
AT = total area difference (or total height difference),
A1 = area (or height) obtained from the first injection, and
A2 = area (or height) obtained from the second injection.
10.3.2 Assuming the ideal gas law, calculate the weight of H2S in the calibration standard by Eq 2:
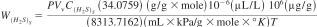
where:
W(H2S)S = weight of H2S in calibration standard, µg,
P = laboratory barometric pressure, kPa,
Vv = volume of headspace vial, mL,
C(H2S)S = concentration of H2S in calibration standard, µL/L,
34.0759 = molecular weight of H2S, g/g x mole,
8313.7162 = (mL x kPa)/(g x mole x °K) = R = ideal gas constant, and
T = laboratory ambient temperature,°K.
If P = 101 325 kPa, Vv = 120 mL, C(H2S)S = 10 µL/L and T = 298.15 °K then:
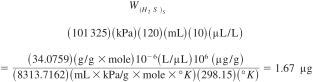
10.3.3 Calculate the response factor from the weight of the H2S in the calibration standard divided by the total area difference obtained for the standard, Eq 3:
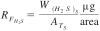
where:
RFH2S = H2S response factor, µg/area, and
ATS = total area or peak height difference from H2S calibration standard analysis.



