ASTM D5769 Standard Test Method for Determination of Benzene, Toluene, and Total Aromatics in Finished Gasolines by Gas Chromatography/Mass Spectrometry
11. Sample Preparation Procedure
11.1 Tare a leak proof sample container (volumetric flask or septum sealed vial). Transfer approximately 10 g of chilled sample and record its mass (Wg) to nearest 0.1 mg. Tare the container and add the internal standards as follows. For the three internal standard version, add a quantity (Ws) of benzene-d6, ethylbenzene-d10, and naphthalene-d8 internal standards at the following approximate mix ratio: 2 mL, 2 mL and 1 g, respectively. For the four internal standard version, add a quantity of benzene-d6, ethylbenzene-d10, napththalene-d8 and toluene-d8 internal standards at the following approximate mix ratio 2 mL, 2 mL, 1 g, and 7 mL, respectively. If each internal standard is added individually, tare the container for each addition, starting with the least volatile internal standard. Record the mass of each internal standard to nearest 0.1 mg.
NOTE 6 - The internal standards may be gravimetrically pre-mixed into a larger stock solution and then added as a single addition and weighing into the samples. The amount of internal standards added must be approximately in the same proportion as that added to the calibration solutions. For example, if 2 g of benzene-d6 was added per 100 mL solutions for calibrations, then add 0.2 g for 10 mL of sample or 0.1 g for 5 mL of sample. The sample solution is then mixed 30 s on a vortex mixer and analyzed by GC/MS, as described previously.
12. Sample Analyses Procedure
12.1 Ensure that the GC/MS operating conditions are identical to those used for calibration, that the system is properly calibrated, and that all of the criteria in Sections 9 and 10 are met.
12.2 Transfer a sufficient quantity of the chilled sample containing the appropriate internal standards from Section 11 to fill a GC autosampler vial and seal with a leak free septum cap.
12.3 Place the sample vial on the autosampler and start the analysis.
13. Calculation
13.1 Mass Concentration of Aromatic Hydrocarbon:
13.1.1 Calibrated Aromatic Components (3.1.5) - Identify the various aromatic components in Table 2 from their retention times and mass spectrum. To identify a compound, obtain a RIC for the primary ion (molecular ion used for quantitation) and the two other major secondary ions listed in Table 7. The criteria below must be met for a qualitative identification:
13.1.1.1 The three characteristic ions for the compound shall be found to maximize in the same or within one spectrum or scan of each other.
13.1.1.2 The ratio of the intensities of the three characteristic ions for the compound must agree within more or less 30 %, more or less 50 % and more or less 100 % relative for ions with a relative intensities of >50 %, 20 to 50 % and <20 %, respectively, when compared to the relative ion intensity ratios obtained for a calibration standard containing the compound at approximately the same concentration.
13.1.1.3 The retention time at the maximum intensity scan in 13.1.1.1 must be within more or less 15 s of the retention time of the authentic compound from the calibration analyses.
13.1.2 Uncalibrated Aromatic Components (3.1.2) - The calibration components in 6.1.2 may not account for all of the aromatic hydrocarbons present in the gasoline sample. Uncalibrated aromatics are identified by the existence of peaks with characteristic ions in specified retention time ranges (see Figs. 2-4). The concentration of the uncalibrated components is estimated using the calibration curves of several of the calibrated components (see 13.1.3).
13.1.3 After the compounds in Table 2 have been properly identified, measure the areas of each peak at the specified primary quantitation mass and that of the internal standards using the same procedure used for the calibration solutions. Using the sample RIC plots in Figs. 2-5, follow the steps below for quantitation.
13.1.3.1 Mass 78 - Use to quantitate benzene from its calibration curve. Ignore any other mass 78 peaks.
13.1.3.2 Mass 92 - Use to quantitate toluene from its calibration curve. Ignore any other mass 92 peaks.
13.1.3.3 Mass 106 - Use to quantitate ethylbenzene and the three xylene isomers from their respective calibrations. 1,3-dimethylbenzene and 1,4-dimethylbenzene may be unresolved or poorly resolved and may be combined for quantitation. Ignore any other mass 106 peaks.
13.1.3.4 Mass 120 - Use to quantitate C9-benzenes using their respective calibration curves. Ignore any other mass 120 peaks.
13.1.3.5 Mass 134 - Use to quantitate C10-benzenes. Assume all mass 134 response up to the retention time of 1,2,3,4-tetramethyl benzene are C10-benzenes. For calibrated components use their corresponding calibration curves. For n-butylbenzene and 1,4-diethylbenzene, which may coelute, use the combined calibration curve of n-butylbenzene and 1,4-diethylbenzene. For the uncalibrated C10-benzenes, sum all of the areas and use the calibration curve of 1,2-diethylbenzene.
13.1.3.6 Mass 117 - Use to quantitate indan and substituted alkylindans. Assume all mass 117 peak eluting after indan are substituted alkyl indans. Use the calibration curve for indan to quantitate all mass 117 peaks.
13.1.3.7 Mass 148 - Use to quantitate C11 benzenes. Use the calibration curve of 1,2-diethylbenzene for quantitation of all detected uncalibrated components with mass 148.
13.1.3.8 Mass 162 - Use to quantitate C12 benzenes. The concentrations of these components may be at or below detection limits. If detected, use the calibration curve of 1,2-diethylbenzene for quantitation of all detected uncalibrated components with mass 162.
13.1.3.9 Mass 128 - Use to quantitate naphthalene from its calibration curve. Ignore all other mass 128 peaks.
13.1.3.10 Mass 142 - Use to quantitate the two methylnaphthalene isomers from their corresponding calibration curves. Ignore all other mass 142 peaks.
13.1.3.11 For the quantitation of the uncalibrated components, DO NOT include the peak areas of any resolved calibrated components having a similar reconstructed (RIC) ion response with the summed areas of the uncalibrated components. The calibrated components are quantitated separately using their respective calibrations.
13.1.4 From the linear least squares fit calibrations, Eq 15, calculate the absolute mass of each aromatic (Wi) in grams in the gasoline samples using the response ratio (rspi) of the areas for the sample of the aromatic component to that of the internal standard as follows:
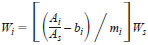
13.1.4.1 If the calibration curves were obtained by forcing the intercept to be zero, then bi = 0.
13.1.4.2 For components that yielded nonlinear calibrations as specified in 9.3.6, calculate Wi using appropriate software.
13.1.5 For the uncalibrated components, either sum all of their peak areas and treat the total area as a single component for quantitation or treat each uncalibrated component as a single component for quantitation and then sum their total concentrations.
NOTE 7 - It may be more appropriate to force the calibration curves used to quantitate the uncalibrated components through the zero intercept, that is, bi = 0, to prevent calculating negative results for the uncalibrated components that are present in the samples at very low concentrations.
13.1.6 To obtain mass % (wi) results for each aromatic hydrocarbon, including uncalibrated aromatics:
wi = (Wi/Wg)(100%)
where:
Wg = mass of gasoline sample
13.1.7 To obtain the mass percent of the total aromatic concentration wt, sum the mass 5 % of each aromatic component, including the mass percent of the uncalibrated components:
Wt = ∑Wi
13.1.8 Report results for total aromatics to nearest 0.1 mass % and for benzene to 0.01 mass %.
13.2 Volumetric Concentration of Aromatics:
13.2.1 Calculate the volumetric concentration according to Eq 18:
vi = wi (Df/Di)
where:
vi = volume % of each aromatic to be determined,
Di = relative density at 60°F (15.56°C) of the individual aromatics as found in Table 2, and
Df = relative density of the fuel under study as determined by Practice D1298 or Test Method D4052.
13.2.2 To obtain the volume percent of the total aromatic concentration Vt, sum the volume percent of each aromatic component, including the volume percent of the uncalibrated components:
vt = ∑vi
13.2.3 Report results for total aromatics to nearest 0.1 volume percent and for benzene to 0.01 volume percent.



