ASTM D5769 Standard Test Method for Determination of Benzene, Toluene, and Total Aromatics in Finished Gasolines by Gas Chromatography/Mass Spectrometry
9. Calibration
9.1 Preparation of Calibration Standards - Multi-component calibration standards using all the compounds listed in Table 1 are prepared by mass according to Practice D4307. The standards may be prepared by combining the specified individual aromatics either into a single mixture or into multiple sets. Multiple sets may be prepared as follows: (1) Set I consists of benzene, methylbenzene (toluene), ethylbenzene, 1,2-dimethylbenzene, 1,3-dimethylbenzene, and 1,4-dimethylbenzene, using 2,2,4-trimethylpentane (isooctane) as a recommended dilution solvent; (2) Set II consists of the remaining C9+ components using a 50/50 mixture of 2,2,4-trimethylpentane and methylbenzene (toluene) as the recommended dilution solvent. Other solvents, such as n-nonane, or co-solvents may be used to improve solubility, chromatographic or mass spectrometric performance, provided these solvents contain no detectable amounts of aromatics which will interfere with the analyses.
NOTE 2 - It may be more convenient to prepare gravimetrically pure (solvent free) batches of Set I and Set II components, which then can be weighed into appropriate diluted standards. The internal standards for Set I are benzene-d6 and ethylbenzene-d10. Toluene-d8 may be added to the internal standard mixture for the quantitation of toluene. The internal standards for Set II are ethylbenzene-d10 and naphthalene-d8.
NOTE 3 - Appropriate internal standards batches may be prepared and then added to calibration standards and samples in a single step.
A minimum of five calibration solutions shall be prepared by mass for single mixtures containing all of the specified calibration compounds. For toluene, three of the calibration standards must be above the 50 % point of the calibration range. If nonlinearity is observed, the addition of a sixth calibration standard is recommended to better define its potential nonlinearity at the higher concentration range. If the calibration solutions are prepared in sets, then for each set, five separate solutions must be prepared over the desired concentration range; for example, five calibration solutions for Set I, and five calibration solutions for Set II. Table 2 gives the recommended volumes to be weighed into 100 mL volumetric flasks or 100 mL septum capped vials for the most concentrated calibration standard. Adjust these concentrations, as necessary, to ensure that the concentrations of the components in the actual samples are bracketed by the calibration concentrations. Solid components are weighed directly into the flask or vial. Other more dilute standards are prepared separately by weighing appropriate amounts of the pure aromatic components. Prepare a calibration standard according to Practice D4307 as follows:
9.1.1 Cap and record the tare weight of the 100 mL volumetric flask or vial to 0.1 mg.
9.1.2 Remove the cap and carefully add an aromatic component to the flask or vial starting with the least volatile component. Cap the flask and record the net mass (Wi) of the aromatic component added to 0.1 mg.
9.1.3 Repeat the addition and weighing procedure for each aromatic component.
9.1.4 If Sets I and II components were pre-mixed gravimetrically, then to each calibration solution, volumetric flask, or vial, weigh appropriate volumes to yield the ten calibration solutions. Calculate the actual mass of each component by multiplying the total mass of the combined mixture by the mass fraction of the individual components in the pre-mixed undiluted mixture.
9.1.5 Similarly add each internal standard and record its net mass (Ws) to 0.1 mg. If standards are prepared in multiple sets; for Set I weigh 2 mL each of benzene-d6 and ethylbenzene-d10, and for Set II weigh 2 mL of ethylbenzene-d10 and 1 g naphthalene-d8.
9.1.6 Dilute to 100 mL total volume the standard with the recommended solvents above or equivalent. It is not necessary to weigh the amount of solvent added since the calculations are based on the absolute masses of the aromatic and internal standard components.
9.1.7 Similarly prepare four additional standards to cover the concentration range of interest. For example, for benzene, prepare 0.1, 0.5, 1.0, 3.0, and 5.0 targeted volume percent standards; for toluene, prepare 2.0, 5.0, 10.0, 15.0, and 19.0 targeted volume percent equivalent standards. If the calibration response for toluene is nonlinear, then add a sixth calibration standard, such as 2.0, 5.0, 10.0, 15.0, 17.0, and 19.0 targeted volume percent.
9.1.8 Store the capped calibration standards in a refrigerator at 0 to 5°C (32 to 40°F) when not in use.
9.1.9 Thoroughly mix the prepared standards using a vortex mixer, or equivalent, and transfer approximately 2 mL of the solution to a vial compatible with the autosampler if such equipment is used. Chill the vials until ready for loading on the autosampler.
NOTE 4 - Highly precise robotic or semi-automated sample preparation systems are available commercially. These systems may be used to prepare mass percent calibration standards and samples for analyses provided that the results for the quality control reference material (Section 10) are met when prepared with the automated systems.
9.2 GC/MS Calibration Procedure:
9.2.1 Prepare the GC/MS system according to manufacturer's instructions and set analysis operating conditions. Table 3 gives suggested operating conditions for split and on-column injection modes.
9.2.2 Before initiating the calibration procedure, tune the mass spectrometer according to manufacturer's instructions. Set the mass spectrometer data system to acquire data in the full scan (TIC-RIC) mode.
9.2.3 The WCOT shall meet the resolution requirements described in 6.1.2 when installed in the GC/MS system.
9.2.4 Prepare a solution of 0.01 mass % of 1,4-diethylbenzene and verify that it is detected with a signal/noise ratio of at least 5 at mass 134.
9.2.5 Inject a solution of 3 mass % of 1,2,3-trimethylbenzene and confirm that the mass spectrometer provides a fragmentation pattern as specified in Table 4.
9.2.6 Sequentially analyze the calibration standards.
9.3 Calibration Calculations:
9.3.1 After the analyses of the calibration standards are complete, integrate the peak area of each calibration component and internal standards using the reconstructed ion chromatogram (RIC) of the characteristic calibration ion listed in Table 1. Obtain the area under the extracted ion at the retention time of the expected aromatic component (or internal standard).
9.3.1.1 Erroneous aromatic concentrations may result when the deuterated internal standards used for calibrations are from a different batch or lot used for the samples. The most accurate results are obtained when using the same batch of internal standards for the calibration and the sample. However, if the ratio of the intensities of (M-1)/M for the internal standards in the calibration standards divided by the ratio of the intensities of (M-1)/M of the corresponding internal standards in the sample being analyzed is less than 0.97 or greater than 1.03 (more or less 3 % relative difference), then use the SUM of M and (M-1) for the total intensities of the deuterated internal standards for quantitation. If the result is within 0.97 to 1.03 or if the same batch of internal standards is used for the calibration standards and the samples, then the molecular ion M of the internal standards may be used for quantitation. Table 1 lists the M and M-1 ions for the specified deuterated internal standards.
9.3.1.2 The deuterated internal standards may show multiple peaks or shoulders due to the resolution of their various deuterated homologues. If this occurs, sum all of the peaks or shoulders, or both.
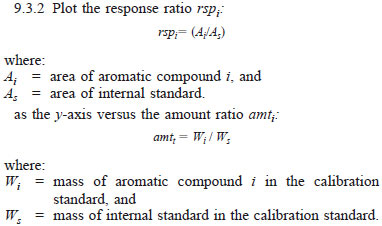
as the x-axis to generate calibration curves for each aromatic component specified in Table 2. See Fig. 1 for an example plot.
9.3.3 Check the correlation r2 value for each aromatic calibration. The value r2 should be at least 0.99 or better and is calculated as follows:
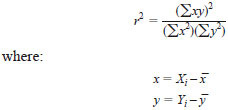

Using the example ideal data set shown in Table 5, r2 would be calculated as follows:
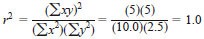
9.3.4 Linear Least Squares Fit - For each aromatic i calibration data set, obtain the linear least squares in the form:
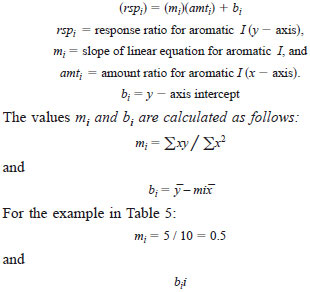
Therefore, the least square equation for the above example in Table 5 is:
(rspi) = 0.5(amti) + 0
NOTE 5 - Normally the bi term is not zero and may be positive or negative. It may be more appropriate to force the calibration through the zero intercept, that is, bi = 0, to prevent calculating negative results for components present in the samples at very low concentrations, such as the uncalibrated components. Software is available on commercial GC/MS systems for performing this. Fig. 1 is an example of a calibration curve forced through the origin with a resulting zero intercept value.
9.3.5 Y-intercept Criteria for Calibrations Curves Not Forced Through Zero - For an optimum calibration, the absolute value of the y-intercept (bi) must be at a minimum, that is, Ai approaches zero when wi is less than 0.1 mass %. As Ai approaches zero, the equation to determine the mass % aromatics i or wi reduces to Eq 14.
wi = (bi/mi)(Ws/Wg)100%
where:
wi = mass % aromatic i
Ws = mass of internal standard added to the gasoline samples for the quantitation of the aromatic component i, g, and
Ws = mass of gasoline samples, g.
Determine the acceptability of the y-intercept of the calibration curve of each aromatic component by substituting in Eq 14 the corresponding slope (mi) and intercept (bi) along with typical (or average) values for sample mass (Wi). If the calculated mass % aromatic i (Wi) is not less than 0.1 mass %, verify the integrity of the GC/MS system and all calibration standards and recalibrate.
9.3.6 Every effort shall be made to obtain linear calibration curves to ensure optimum chromatographic performance and to ensure that the MS signal is not saturated. However, for certain components present in large concentrations in the samples, such as toluene, some nonlinearity may exist. For such compounds the following quantitation procedure must be used:
9.3.6.1 Plot component concentration versus compound response factor of each calibration standard.
9.3.6.2 If the deviation of the response factors for the higher concentration standards is less than 5 % relative than the response factors of the lower concentration standards that are in the linear range, then use a linear fit for all calibration points.
9.3.6.3 If the deviation in 9.3.6.2 is in the range of 5 to 10 % relative, then use a quadratic fit.
9.3.6.4 If the deviation in 9.3.6.2 is greater than 10 % relative, then no samples can be analyzed until the deviation is corrected.
9.3.7 The GC/MS system shall be recalibrated whenever results of the quality control reference material do not agree within the tolerance levels specified in 10.1.

