10. Calculation and Report
10.1 Calculate the mass % oxygen for the QC standard and samples as follows:
Mass % Oxygen = (R x K)/(M x r)
where:
R = blank corrected instrument response,
K = K-factor, refer to Eq 1, assume unity for Test Method D,
M = mass of sample, mg, = volume (µL) x density (g/mL), and
r = recovery, refer to 9.4.3, assume unity for Test Methods A, B, and C.
10.2 For instruments with computer data systems, the calculation of the K-factor (Eq 1) and the calculation of mass % oxygen (Eq 2) can be automatic with a digital readout provided.
10.3 Report mass % oxygen to the nearest 0.01 %.
11. Precision and Bias
11.1 Precision - The precision of these test methods was determined by statistical analysis of interlaboratory test results. Twelve laboratories analyzed in duplicate eight different samples, providing a total of thirteen data sets. One laboratory used two different test methods. The breakdown on data sets by test method is: Test Method A, three; Test Method B, two; Test Method C, three; Test Method D, five. The statistical analysis was performed on the set of 13 data sets because the reductive pyrolysis technique is common to all four test methods. Separate statistics were not determined for individual test methods. The sample set included anhydrous methanol and gasoline stocks that were spiked with one or more of the following: isobutanol, n-butanol, sec-butanol, tert-butanol, di-isopropyl ether, ethanol, ethyl tert-butyl ether, methanol, methyl tert-butyl ether, n-propanol, isopropanol, tert -amyl methyl ether.
11.1.1 Repeatability - The difference between two test results, obtained by the same operator with the same apparatus under constant operating conditions on identical test materials would, in the long run, in the normal and correct operation of the test method, exceed the following values in only one case in twenty.
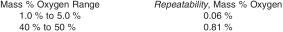
11.1.2 Reproducibility - The difference between two single and independent results, obtained by different operators working in different laboratories on identical test materials, would in the long run, in the normal and correct operation of the test method, exceed the following values in only one case in twenty.

11.2 Bias - Bias was determined from interlaboratory results obtained on NIST SRM 1838, which contains ethanol. The null hypothesis that was tested was that the true difference between the grand average result and the NIST certified value is zero. The result of the hypothesis testing was that if the true difference was zero, the determined difference would occur by chance approximately 50 % of the time. Hence, the null hypothesis of no difference or no bias is accepted.
12. Keywords
12.1 carbon dioxide; carbon monoxide; di-isopropyl ether; ethanol; ethyl tert-butyl ether; isobutanol; isopropanol; methanol; methyl tert-butyl ether; n-butanol; n-propanol; oxygen; reductive pyrolysis; sec-butanol; tert-butanol; tert-amyl methyl ether

