10. Procedure
10.1 Weigh 10 g (to 0.1 mg) of the dried coke prepared in 8.2 into a labeled preignited platinum dish. (Warning - In addition to other precautions, to minimize the potential of contamination, prepare the platinum ware by boiling in dilute HCl (5 volume % HCl plus 95 % water) rinsing thoroughly with a reagent-grade water. After this initial cleaning, handle the platinum ware with clean tongs, and protect from all sources of contamination. Clean and protect all the glassware used in analyses.)
10.2 Place the platinum dish in a cold muffle furnace and heat directly to 525°C with the furnace door opened approximately 7 mm to allow exchange of combustion gases and air until all carbonaceous matter is removed. Transfer the platinum dish to a dessicator and cool to room temperature.
10.3 To convert the ash into a solution, weigh on an analytical balance onto a tared weighing paper, 1 g (+/- 5 mg 200 +/- 10°C) of Li2B4O7 powder. Mix the ash and lithium tetraborate by sprinkling Li2B4O7 evenly over the ash. Place the platinum dish onto a ceramic triangle resting on a ring stand over a Meker type burner and adjust the forced air gas flame so that the Li2B4O7 melts in about 30 s. Using the platinum-tipped tongs, gently swirl the melt to dissolve the ash. Continue heating over the burner for 2 to 3 min or until a clear, transparent melt is obtained. Alternatively, heat in a furnace at 950 +/- 10°C for 10 min or until the Li2B4O7 melts.
NOTE 3 - The ideal fusion after cooling will look like a clear glass inside the platinum dish. An opaque melt indicates poor fusion and some of the ash may remain insoluble during the dissolution step.
10.4 Allow the melt to cool for 5 to 10 min on a silica plate. Add a 25.4 mm (1 in.) TFE-fluorocarbon coated magnetic stirring bar, and 25 mL of Solution 1, and place immediately on the stirring hot plate. Heat the solution to just below boiling temperature and maintain for not more than 30 min with constant stirring, until the melt has completely dissolved.
NOTE 4 - If the stirring is not constantly maintained, some of the ash constituents may precipitate, primarily hydrous silica, due to heating the highly acidic solution. If this occurs, it is necessary to repeat the analysis.
10.5 Remove the dish from the hot plate, rinse down the walls of the dish with water, and quantitatively transfer the solution to a 100-mL flask. Add 10 mL of Solution 2, dilute with water, and mix thoroughly (see Note 4).
10.6 Prepare any required dilution using Solution 3 (7.7), diluted 1:1 with water, as the dilutent.
NOTE 5 - Lanthanum is included in the solution as a releasing agent for calcium and as an ionization suppressant for aluminum and vanadium.
10.7 Establish the AAS operating conditions (see Section 9). Select the flame gases and spectral lines from the requirements presented in Table 2.
NOTE 6 - Each analyst determines the sensitivity and linear range of calibration of his own equipment and chooses concentration ranges for standards compatible with the samples and instrument specific to his own work. Sample dilutions can be required for the determination of some elements. Table 2 lists the oxidant gases used in the analyses for determining the precision of this method. However, nitrous oxide can be used as the oxidant for all of the elements of interest to reduce errors due to chemical interferences.
10.8 Prepare calibration standards, including a calibration blank, using 50 mL of Solution 3 per 100 mL. Dilute with water (see Note 5).
NOTE 7 - Standard and sample solutions are of similar composition to minimize errors due to matrix effects.
10.9 Using the AAS, determine the concentration of each metal in the sample solution. Standards must bracket the sample concentration.
11. Calculation
11.1 Calculate parts per million (milligram per kilogram) of each metal in the sample as follows:
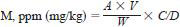
where:
M = metal,
A = metal in solution analyzed, ppm (mg/L),
V = volume of sample solution, mL,
W = weight of sample, g, and
C/D = dilution factor (amount of dilution of the sample solution in 10.6 needed to bring the metal concentrations into the range of standard solutions).
11.2 Calculations used and results reported are on a dried original sample basis.

