TEST METHOD B - COMBUSTION AND MICROCOULOMETRY
18. Apparatus
18.1 Combustion Furnace - The sample specimen is to be oxidized in an electric furnace capable of maintaining a temperature of 800°C to oxidize the organic matrix.
18.2 Combustion Tube - Fabricated from quartz and constructed so a sample, which is vaporized completely in the inlet section, is swept into the oxidation zone by an inert gas where it mixes with oxygen and is burned. The inlet end of the tube shall hold a septum for syringe entry of the sample and side arms for the introduction of oxygen and inert gases. The center section is to be of sufficient volume to ensure complete oxidation of the sample.
18.3 Titration Cell - Containing a sensor-reference pair of electrodes to detect changes in silver ion concentration and a generator anode-cathode pair of electrodes to maintain constant silver ion concentration and an inlet for a gaseous sample from the pyrolysis tube. The sensor, reference, and anode electrodes shall be silver electrodes. The cathode electrode shall be a platinum wire. The reference electrode resides in a saturated silver acetate half-cell. The electrolyte contains 70 % acetic acid in water.
18.4 Microcoulometer, having variable gain and bias control, and capable of measuring the potential of the sensing-reference electrode pair, and of comparing this potential with a bias potential, and of applying the amplified difference to the working-auxiliary electrode pair so as to generate a titrant. The microcoulometer output signal shall be proportional to the generating current. The microcoulometer may have a digital meter and circuitry to convert this output signal directly to nanograms or micrograms of chloride.
18.5 Sampling Syringe - A microlitre syringe of 50-µL capacity capable of accurately delivering 5 to 50 µL of sample into the pyrolysis tube. A 3- or 6-in. (76.2- or 152.4-mm) needle is recommended to reach the inlet zone of approximately 500°C in the combustion zone.
18.6 A constant rate syringe pump or manual dispensing adaptor may be used to facilitate slow injection of the sample into the combustion tube. It is recommended that the injection rate not exceed 0.5 µL/s.
19. Reagents and Materials
19.1 Acetic Acid, glacial acetic acid. (Warning - Corrosive, causes severe burns.)
19.2 Argon, Helium, Nitrogen, or Carbon Dioxide, high purity grade (HP) used as the carrier gas. (Warning - These gases are normally stored in cylinders under high pressure. These gases also dilute the oxygen content of the surrounding air when they leak.)
19.3 Cell Electrolyte Solution, 70 % acetic acid, combine 300 mL reagent water (see 6.2) with 700 mL acetic acid (see 19.1) and mix well.
19.4 Chloride, Standard Stock Solution, 1000 mg chloride per litre. Accurately dispense 1.587 g of chlorobenzene into a 500-mL volumetric flask and dilute to volume with 2,2,4,trimethyl pentane (isooctane).
NOTE 2 - The exact concentration of chloride may be determined by multiplying the mass of chlorobenzene by the product of the atomic mass of chlorine divided by the molecular mass of chlorobenzene and then multiplying that result by 2000.
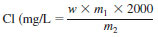
where:
w = mass of chlorobenzene weighed,
m1 = atomic mass of chlorine, and
m2 = molecular mass of chlorobenzene.
19.5 Chlorine, Standard Solution, 10 mg chloride per litre. Pipet 1.0 mL of chloride stock solution (see 19.4) into a 100-mL volumetric flask and dilute to volume with 2,2,4,trimethyl pentane (isooctane).
19.6 Chlorobenzene, reagent grade.
19.7 Gas Regulators, two-stage gas regulator must be used on the reactant and carrier gas.
19.8 Isooctane, 2,2,4-trimethylpentane, reagent grade.
19.9 Oxygen, high purity grade, used as the reactant gas.
19.10 Silver Acetate, powder purified for saturated reference electrode.
20. Preparation of Apparatus
20.1 Set up the analyzer in accordance with the equipment manufacturer instructions.
20.2 The typical operational conditions are as follows:
Reactant gas flow, O2: 160 mL/min
Carrier gas flow: 40 mL/min
Furnace temperature:
Inlet zone: 700°C
Center and outlet zones: 800°C
Coulometer:
Bias voltage, mV: 240 - 265
Gain: ca. 1200
20.3 Optimize the bias voltage setting for the titration cell null-point by injecting 30 µL of chloride-free water directly into the titration cell using a 6-in. needle. Adjust bias up or down to minimize the total integrated value due to this dilution effect.
21. Procedure
21.1 Fill a 50-µL syringe with about 30 to 40 µL of the sample of washed naphtha fraction of crude oil, being careful to eliminate bubbles. Then retract the plunger so that the lower liquid meniscus falls on the 5-µL mark, and record the volume of liquid in the syringe. After the sample has been injected, again retract the plunger so that the lower liquid meniscus falls on the 5-µL mark, and record the volume of liquid in the syringe. The difference in the two volume readings is the volume of sample injected.
21.2 Alternately, obtain the sample injection device mass before and after injection to determine the amount of sample injected. This method provides greater precision than the volume delivery method, provided a balance with a precision of +/- 0.01 mg is used and the syringe is carefully handled to obtain repeatable weighings.
21.3 Inject the sample into the pyrolysis tube at a rate not to exceed 0.5 µL/s.
21.4 Below 5 µg/g, the needle-septum blank will become increasingly more obvious. To improve precision, insert the syringe needle into the hot inlet and then wait until the needle-septum blank is titrated before injecting the sample or standard.
21.5 For specimens containing more than 25 µg/g Cl only 5.0 µL of sample need be injected.
21.6 Verify the system recovery, the fraction of chlorine in the standard that is titrated, every 4 h by using the standard solution (see 19.5). System recovery is typically 85 % or better.
21.7 Repeat the measurement of the calibration standard at least three times.
21.8 Check the system blank daily with reagent grade isooctane (see 19.8). Subtract the system blank from both sample and standard data. The system blank is typically less than 0.2 µg/g chloride once the needle-septum blank has been titrated (see 21.4).
22. Calculation
22.1 Calculate chloride concentration in the naphtha fraction as follows:
22.1.1 For microcoulometers, which read directly in nanograms of chloride, the following equations apply:
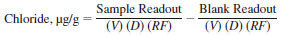
or

where:
Readout = displayed integrated value (sample/standard/blank),
V = volume injected µL,
D = density, g/mL (11.3),
RF = recovery factor, ration of chloride determined in standard divided by known standard content minus the system blank.
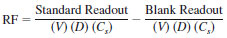
M = mass of sample specimen, mg, and
Cs = concentration of standard, mg/L
22.1.2 For microcoulometers with only analog signal output to a recorder the following equation applies:
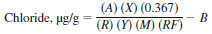
where:
A = area in appropriate units,
X = recorder sensitivity for full-scale response, mV,
0.367 =
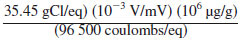
R = resistance, Ω,
Y = area equivalence for a full-scale response on the recorder per second-area units per second,
M = mass of sample, g,
RF = recovery factor, and
B = system blank, µg/g Cl.
22.2 The concentration of organic chloride in the original crude oil sample specimen can be obtained by multiplying the chloride concentration in the naphtha fraction (see 22.1) by the naphtha fraction (see 12.1).

