TEST METHOD A - SODIUM BIPHENYL REDUCTION AND POTENTIOMETRY
13. Apparatus
13.1 Electrodes - The cleaning and proper care of electrodes are critical to the accuracy of this test. Manufacturer's instructions for the care of electrodes shall be followed.
13.1.1 Glass, general purpose. When glass electrodes are in continuous use, weekly cleaning with chrome-sulfuric acid (Warning - Strong oxidizer; can cause severe burns; recognized carcinogen), or other strongly oxidizing cleaning solution, is recommended.
13.1.2 Silver-Silver Chloride, billet-type.
13.2 Titrator, potentiometric. The titrator is equipped with a 5-mL or smaller buret and a magnetic stirring motor.
14. Reagents and Materials
14.1 Acetone, chloride-free. (Warning - Extremely flammable, can cause flash fires. Health hazard.)
14.2 Congo Red Paper.
14.3 2,2,4, trimethyl pentane (isooctane), reagent grade. (Warning - Flammable. Health hazard.)
14.4 Nitric Acid, approximately 5 M. (Warning - Corrosive, causes severe burns.) Add 160 mL of concentrated nitric acid to about 200 mL of water and dilute to 500 mL.
14.5 2-Propanol, chloride-free. (Warning - Flammable. Health hazard.)
14.6 Silver Nitrate, 0.01 M, standard aqueous solution.
14.7 Sodium Biphenyl Reagent - This is packed in 0.5-oz French square bottles (hereafter referred to as vials). The entire contents of one vial are used for each analysis. One vial contains 13 to 15 meq of active sodium. Store the sodium biphenyl reagent in a cool storage area, but do not refrigerate. Prior to using, warm the reagent to approximately 50°C and shake thoroughly to ensure a homogeneous liquid.
14.8 Toluene, chloride-free. (Warning - Flammable. Health hazard.)
15. Preparation of Apparatus
15.1 Recoating Silver-Silver Chloride Electrodes - Clean the metal surfaces of a pair of silver-silver chloride electrodes with mild detergent and scouring powder. Rinse the electrodes in distilled water. Immerse the metallic tips in saturated potassium chloride solution. Connect one electrode to the positive pole of a 1.6-V battery and the other to the negative pole. Reverse the polarity for several intervals of a few seconds each to alternately clean and recoat the receptor electrode (connected to the positive pole). When adequately coated, the receptor electrode tip will turn violet in color. This results from the action of light on the fresh silver chloride.
16. Procedure
16.1 Use extreme care to prevent contamination. Reserve all glassware for the chloride determination. Rinse glassware with distilled water followed by acetone just prior to use. Avoid using chlorine-containing stopcock greases such as chlorotrifluoroethylene polymer grease.
16.2 Place 50 mL of toluene in a 250-mL separatory funnel and add the contents of one vial of sodium biphenyl reagent. Swirl to mix and add about 30 g, obtaining the mass to the nearest 0.1 g of the washed naphtha fraction of crude oil sample. Obtain the mass of the sample bottle to determine the exact amount taken. Stopper the separatory funnel and swirl to mix the contents thoroughly. The solution or suspension that results should be blue-green in color. When it is not, add more sodium biphenyl reagent (one vial at a time) until the solution or suspension is blue-green.
16.3 Allow 10 min after mixing for the reaction to be completed, then add 2 mL of 2-propanol and swirl gently with the funnel unstoppered for a time until the blue-green color changes to white, indicating that no free sodium remains. Stopper the funnel and rock it gently, venting pressure frequently through the stopcock. Then add 20 mL of water and 10 mL of 5 M nitric acid. Shake gently, releasing the pressure frequently through the stopcock. Test the aqueous phase with Congo red paper. If the paper does not turn blue, add additional 5 M nitric acid in 5-mL portions until the blue color is obtained.
16.4 Drain the aqueous phase into another separatory funnel containing 50 mL of isooctane and shake well. Drain the aqueous phase into a 250-mL titration beaker. Make a second extraction of the sample-and-isooctane mixture with 25 mL of water that has been acidified with a few drops of 5 M nitric acid. Add this second extract to the 250-mL titration beaker. Evaporate the solution on a hot plate kept just below the boiling point of the liquid until 25 to 30 mL remains. Do not boil or evaporate to less than 25 mL as loss of chloride may occur.
16.5 Cool the solution and add 100 mL of acetone. Titrate the solution potentiometrically with standard 0.01 M silver nitrate, using glass versus silver-silver chloride electrodes. If an automatic titrator, such as a Metrohm, is available, use the semi-micro 5-mL piston buret. If the titration is carried out with a manually-operated pH meter, use a 5-mL semi-micro buret that can be estimated to three decimal places in millilitres.
16.6 Determine the endpoint for the manual titration by plotting the data showing emf versus volume of silver nitrate solution used. Determine the endpoint for the automatic titrator from the midpoint of the inflection of the titration curve.
16.7 Determine a blank for each group of test specimens by using all of the reagents, including the sodium biphenyl, and following all the operations of the analysis except that the sample itself is omitted.
17. Calculation
17.1 Calculate chloride concentration in the naphtha fraction as follows:
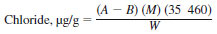
where:
A = volume of titrant for the sample specimen, mL,
B = volume of titrant for the blank, mL,
M = molarity of silver nitrate, and
W = mass of sample specimen, g.
17.2 The concentration of organic chloride in the original crude oil sample specimen can be obtained by multiplying the chloride concentration in the naphtha fraction (see 17.1) by the naphtha fraction (see 12.1).

