3. Summary of Test Method
3.1 A crude oil distillation is performed to obtain the naphtha cut at 204°C (400°F). The distillation method was adapted from Test Method D86 for the distillation of petroleum products. The naphtha cut is washed with caustic, repeatedly when necessary, until all hydrogen sulfide is removed. The naphtha cut, free of hydrogen sulfide, is then washed with water, repeatedly when necessary, to remove inorganic halides (chlorides).
3.2 There are two alternative test methods for determination of the organic chloride in the washed naphtha fraction, as follows.
3.2.1 Test Method A, Sodium Biphenyl Reduction and Potentiometry - The washed naphtha fraction of a crude oil specimen is weighed and transferred to a separatory funnel containing sodium biphenyl reagent in toluene. The reagent is an addition compound of sodium and biphenyl in ethylene glycol dimethyl ether. The free radical nature of this reagent promotes very rapid conversion of the organic halogen to inorganic halide. In effect this reagent solubilizes metallic sodium in organic compounds. The excess reagent is decomposed, the mixture acidified, and the phases separated. The aqueous phase is evaporated to 25 to 30 mL, acetone is added, and the solution titrated potentiometrically.
3.2.2 Test Method B, Combustion and Microcoulometry - The washed naphtha fraction of a crude oil specimen is injected into a flowing stream of gas containing about 80 % oxygen and 20 % inert gas, such as argon, helium, or nitrogen. The gas and sample flow through a combustion tube maintained at about 800°C. The chlorine is converted to chloride and oxychlorides, which then flow into a titration cell where they react with the silver ions in the titration cell. The silver ions thus consumed are coulometrically replaced. The total current required to replace the silver ions is a measure of the chlorine present in the injected samples.
3.2.3 The reaction occurring in the titration cell as chloride enters is as follows:
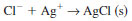
3.2.4 The silver ion consumed in the above reaction is generated coulometrically thus:

3.2.5 These microequivalents of silver are equal to the number of microequivalents of titratable sample ion entering the titration cell.
4. Significance and Use
4.1 Organic chloride species are potentially damaging to refinery processes. Hydrochloric acid can be produced in hydrotreating or reforming reactors and the acid accumulates in condensing regions of the refinery. Unexpected concentrations of organic chlorides cannot be effectively neutralized and damage can result. Organic chlorides are not known to be naturally present in crude oils and usually result from cleaning operations at producing sites, pipelines, or tanks. It is important for the oil industry to have common methods available for the determination of organic chlorides in crude oil, particularly when transfer of custody is involved.
5. Interferences
5.1 Test Method A - Other titratable halides will also give a positive response. These titratable halides include HBr and HI.
5.2 Test Method B - Other titratable halides will also give a positive response. These titratable halides include HBr and HI (HOBr and HOI do not precipitate silver). Since these oxyhalides do not react in the titration cell, approximately 50 % microequivalent response is detected.
5.2.1 This test method is applicable in the presence of total sulfur concentration of up to 10 000 times the chlorine level.
6. Purity of Reagents
6.1 Purity of Reagents - Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination.
6.2 Purity of Water - Unless otherwise indicated, references to water shall be understood to mean reagent water as defined by Type III of Specification D1193.

