TEST METHOD A - LIQUID-LIQUID EXTRACTION
8. Scope
8.1 This test method covers the estimation of oil and grease from 4 to 100 mg/L in water and wastewater by a gravimetric determination of fluorocarbon extractable substances from an acidified sample.
8.2 This test method is generally applicable to samples containing extractable substances.
9. Summary of Test Method
9.1 In this test method, an acidified water sample is extracted with fluorocarbon solvent in a separatory funnel.
9.2 In the gravimetric portion of the procedure, the fluorocarbon solvent containing the extracted materials is evaporated and the residue is determined gravimetrically.
10. Apparatus
10.1 Drying Oven, 103°C.
10.2 Evaporating Flask, 250-mL capacity. A flat-bottom boiling flask with standard taper fitting is recommended to facilitate solvent recycling.
10.3 Distillation Apparatus, water-cooled condenser, side arm, and receiver, all of appropriate standard taper fitting.
10.4 Separatory Funnels, 2–L funnels with TFE-fluorocarbon stopcocks.
10.5 Steam Bath.
10.6 Desiccator.
11. Reagents and Materials
11.1 Acetone ((CH3)2CO), technical grade.
11.2 Filter Paper, any high flow, low-retention grade, or optionally phase separating paper.
11.3 Fluorocarbon Solvent (Chlorofluorocarbon-113 or 1,1,2-Trichloro-1, 2,2-Trifluoroethane) must be shown to contain no significant residue on evaporation. Redistill if necessary.
11.4 Hydrochloric Acid (HCl), sp gr 1.19.
11.5 Sodium Bisulfate - (NaHSO4·H2O).
11.6 Sodium Chloride (NaCl).
11.7 Sodium Sulfate (Na2SO4), anhydrous.
11.8 Sulfuric Acid (H2SO4), sp gr 1.84.
12. Procedure
12.1 Tare, to the nearest tenth of a milligram, a boiling flask that has been dried at 103°C in an oven for 1 h and cooled in a desiccator to room temperature. Caution - Always handle the flask with metal tongs or weighing gloves to avoid deposition of body oils.
NOTE 3 - Run a reagent and materials blank to show that they contain no significant residue with respect to the precision of the test method at the level measured.
NOTE 4 - Frequently, solvent will extract plasticizer from plastic tubing that is used to transfer from one container to another and from shipping container liner. Check for contamination by evaporating 180 mL of solvent on a steam bath and weighing the residue. The solvent should leave no measurable residue greater than 0.1 mg. If this value is exceeded, distill the solvent and check the distillate for residue.
12.2 Mark the sample bottle at the water meniscus for later determination of sample volume. Pour the acidified sample into a separatory funnel.
12.3 Add 60 mL of fluorocarbon solvent to the sample bottle, cap, and shake the bottle well. Pour the solvent into the separatory funnel and extract the sample by shaking vigorously for 2 min. Invert the separatory funnel and vent with stopcock to relieve pressure buildup during the extraction. After the layers have separated, drain the solvent layer through filter paper held by a small funnel into the tared boiling flask. If emulsion problems are anticipated, add 1 g Na2SO4 to the filter paper cone and slowly drain the solvent through the crystals. Add more Na2SO4 if necessary.
NOTE 5 - Solvent phase separation paper helps to keep water out.
12.4 If a clear solvent layer cannot be obtained due to emulsion with water, add up to 100 g of NaCl to separatory funnel. Shake to dissolve the salt. Frequently this will break the emulsion. If the emulsion cannot be broken, this type of sample must be analyzed by the Soxhlet extraction test method.
12.5 Repeat the bottle rinse and extraction with two additional 60-mL portions of solvent, combining all solvent in the flask. Rinse the filter with 20 mL of solvent, into the flask.
12.6 Proceed to 13.1.
13. Procedure, Gravimetric for Test Methods A and B
13.1 Evaporate the solvent from the boiling flask (12.5, 20.8) on a hot water bath or steam bath. (Recovering solvent using a condenser system is recommended.)
13.2 When only a few millilitres of solvent remain (under 10 mL of solvent is not recommended), leave the flask on the steam bath and draw air through the flask using vacuum for 5 min to remove the last traces of solvent or residual water. Carefully wipe the exterior of the flask with a lint free cloth and a small amount of acetone to remove any water adhering to the flask. Warning - Wear protective gloves (polyethylene or similar solvent-resistant material) to prevent the acetone from coming in contact with the skin. Use acetone in a fume hood.
13.3 Place in a desiccator for 1 h, remove, and weigh immediately, to the nearest tenth of a milligram.
13.4 Measure the original sample volume by filling the sample bottle to the mark with water at 20°C and measure the volume of water with a graduated cylinder, to the nearest 5 mL.
NOTE 6 - It is recommended that the chlorofluorocarbon-113 solvent be recycled to reduce costs and minimize discharges to the atmosphere. To recycle the solvent, attach a water cooled condenser with a side arm take off. The recovered solvent can usually be reused for this test without redistillation.
14. Calculation
14.1 Calculate the results of the determination, in milligrams per litre as follows:
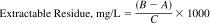
where:
A = tare weight of boiling flask, mg,
B = weight of boiling flask after removal of extraction solvent, mg, and
C = volume of sample, L.
Report results to the nearest milligram per litre.
15. Precision and Bias
15.1 Nine operators from nine laboratories determined four concentration levels of oil and grease in reagent water, Type IV, over three days.
15.2 Recoveries of known amounts of oil and grease in a series of prepared standards were as shown in Table 1.
15.3 It should be recognized that these data may not apply to different water matrices.

