6. Procedure
6.1 Calculate the molar volume of the lubricant as follows:
6.1.1 Determine the density at 298 K by Practice D1298 or equivalent. If the density at 293 K is known (as required for Test Method D3238) multiply it by 0.996 to obtain d with sufficient accuracy.
6.1.2 Determine the molecular weight by Test Method D2502.
6.1.3 Calculate the molar volume as follows:
V = M/d
6.2 Calculate the dispersion parameter by these steps:
6.2.1 Determine the refractive index at 298 K by Test Method D1218.
If a value at 293 K is known (as required for Test Method D3238) multiply it by 0.998 to obtain nD with sufficient accuracy.
6.2.2 Calculate the refractivity function as follows:
y = (nD2 - 1)/(nD2 + 2)
6.2.3 Calculate the parameter as follows:
δd = 45y3 - 119y2 + 108y - 4.58
6.3 For hydrocarbons, calculate δd and P as follows:
6.3.1 Determine CA and CN by Test Method D3238.
6.3.2 Calculate the parameters as follows:
P = 0.0143CA and
H = 0.0286CA + 0.0143CN
6.4 For esters, calculate P and H as follows:
6.4.1 Determine the saponification number by Test Method D94.
6.4.2 Calculate the parameter as follows:
P = 0.00815 Sd and
H = 0.00173 SM/V0.5
6.5 Calculate the volume fraction of water at 298 K and saturation as follows:
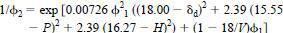
NOTE 1 - For hydrocarbons, it may be safely assumed that Φ1 = 1.00. However, that can introduce a significant error for some esters, so calculate Φ2 stepwise. Start with Φ1 = 1.00, next step Φ1 = 1 - Φ2 from the first step, and so on until no further significant change is noted.
6.5.1 Rarely are more than three steps needed to obtain constancy to three significant figures. A small programmable calculator, which is strongly recommended for the whole procedure, can readily be set into the interative cycle described.
6.6 Calculate the mole fraction at 298 K as follows:
x = V2/18
6.7 Calculate the mole fraction at temperature T as follows:
xT = exp ((625 - T)/1.097) ln x)
6.8 Calculate the solubility by weight, using
G = 18 x 10(6)xT/M(1 - xT)
6.9 If the system was not saturated at equilibrium, with at least a trace of liquid water present, multiply xT by RH/100; then convert to G as before. (Unless G is larger than 1000, this adjustment can be made directly on it.)
6.10 Multiply G by RH/100, if the system was not saturated, with at least a trace of liquid water present. If G is greater than 1000, multiply xT by RG/100 before converting to G.



