ASTM D4052 standard test method for density, relative density, and API gravity of liquids by digital density meter
9. Preparation of Apparatus
9.1 Set up the density analyzer (including the constant
temperature bath and related attachments, if necessary) following the manufacturer's instructions. Adjust the bath or internal temperature control so that the desired test temperature is established and maintained in the sample compartment of the analyzer. Calibrate the instrument at the same temperature at which the density or relative density of the sample is to be measured or perform an adjustment (see 3.1.1.1 Discussion) in preparation of analyzing samples. (Warning - Precise setting and control of the test temperature in the sample tube is extremely important. An error of 0.1°C can result in a change in density of one in the fourth decimal when measuring in units of g/mL.)
10. Calibration of Apparatus
10.1 As a minimum requirement, calibration of the instrument is required when first set up, whenever the test temperature is changed (unless the instrument is capable of performing an adjustment; see 3.1.1.1 Discussion), or as dictated by quality control (QC) sample results (see 11.1).
10.2 When calibration of the instrument is required, it is necessary to calculate the values of the constants A and B from the periods of oscillation (T) observed when the sample cell contains air and redistilled, freshly boiled and cooled reagent water. Other calibrating materials such as n-nonane, n-tridecane, cyclohexane, and n-hexadecane (for high temperature applications) can also be used as appropriate, provided the reference materials have density values that are certified and traceable to national standards.
NOTE 2 - On certain newer, commercially available instruments, a viscosity correction feature may be available and utilized in density determinations to minimize potential biases. Refer to information in the Section 15 for more specifics.
10.2.1 While monitoring the oscillator period, T, flush the sample tube with cleaning solvent, followed with an acetone flush and dry with dry air. Contaminated or humid air can affect the calibration. When these conditions exist in the laboratory, pass the air used for calibration through a suitable purification and drying train. In addition, the inlet and outlet ports for the U-tube must be plugged during measurement of the calibration air to prevent ingress of moist air.
10.2.2 Allow the dry air in the U-tube to come to thermal equilibrium with the test temperature and record the T-value for air.
10.2.3 Introduce a small volume (about 1 to 2 mL) of redistilled, freshly boiled and cooled reagent water into the sample tube using a suitable syringe or alternate, as described in 6.4 and 6.5. The test portion must be homogeneous and free of even the smallest air or gas bubbles. Allow the display to reach a steady reading and record the T-value for water.
10.2.4 Calculate the density of air at the temperature of test using the following equation:
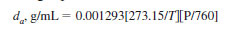
where:
T = temperature, K, and
P = barometric pressure, torr.
10.2.5 Determine the density of water at the temperature of test by reference to Table 1.
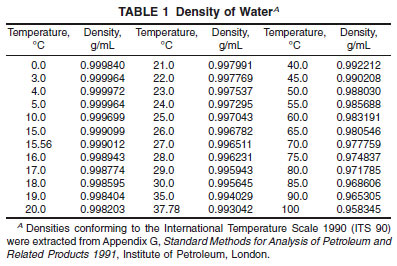
10.2.6 Using the observed T-values and the reference values for water and air, calculate the values of the Constants A and B using the following equations:
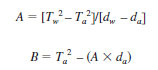
Tw = observed period of oscillation for cell containing water,
Ta = observed period of oscillation for cell containing air,
dw = density of water at test temperature, and
da = density of air at test temperature.
10.2.6.1 Alternatively, use the T and d values for the other reference liquid if one is used.
10.2.7 If the instrument is equipped to calculate density from the constants A and B and the observed T-value from the sample, then enter the constants in the instrument memory in accordance with the manufacturer's instructions. Alternatively, if the instrument is equipped to do so, let it make the appropriate corrections in the calibration or adjustment constants as part of the built in calibration or adjustment procedure.
10.2.8 Check the calibration and adjust if needed by performing the routine calibration check described in 10.3.
10.2.9 To calibrate the instrument to display relative density, that is, the density of the sample at a given temperature referred to the density of water at the same temperature, follow 10.2.1-10.2.7, but substitute 1.000 for dw in performing the calculations described in 10.2.6.
10.3 On some density meter analyzers, weekly calibration adjustments to constants A and B can be made if required, without repeating the calculation procedure. The need for a change in calibration is generally attributable to deposits in the sample tube that are not removed by the routine flushing procedure. Although this condition can be compensated for by adjusting A and B, it is good practice to clean the tube with a strong oxidizing acid (Warning - Causes severe burns) or surfactant cleaning fluids whenever a major adjustment is required.
10.3.1 Flush and dry the sample tube as described in 10.2.1 and allow the display to reach a steady reading. If the display does not exhibit the correct density for air at the temperature of test, repeat the cleaning procedure or adjust the value of constant B commencing with the last decimal place until the correct density is displayed.
10.3.2 If adjustment to constant B was necessary in 10.3.1 then continue the recalibration by introducing redistilled, freshly boiled and cooled reagent water into the sample tube as described in 10.2.3 and allow the display to reach a steady reading. If the instrument has been calibrated to display the density, adjust the reading to the correct value for water at the test temperature (Table 1) by changing the value of constant A, commencing with the last decimal place. If the instrument has been calibrated to display the relative density, adjust the reading to the value 1.0000.
NOTE 3 - If performing a weekly calibration adjustment, it can be found that more than one value each for A and B, differing in the fourth decimal place, will yield the correct density reading for the density of air and water. The setting chosen would then be dependent upon whether it was approached from a higher or lower value. The setting selected by this method could have the effect of altering the fourth place of the reading obtained for a sample.
10.4 Some analyzer models are designed to display the measured period of oscillation only (T-values) and their calibration requires the determination of an instrument constant K, which must be used to calculate the density or relative density from the observed data.
10.4.1 Flush and dry the sample tube as described in 10.2.1 and allow the display to reach a steady reading. Record the T-value for air.
10.4.2 Introduce redistilled, freshly boiled and cooled reagent water into the sample tube as described in 10.2.3, allow the display to reach a steady reading and record the T-value for water.
10.4.3 Using the observed T-values and the reference values for water and air (10.2.4 and 10.2.5), calculate the instrument constant K using the following equations:
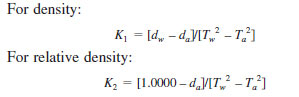
where:
Tw = observed period of oscillation for cell containing water,
Ta = observed period of oscillation for cell containing air,
dw = density of water at test temperature, and
da = density of air at test temperature.



