ASTM D4047 Standard Test Method for Phosphorus in Lubricating Oils and Additives by Quinoline Phosphomolybdate Method
8. Procedure
8.1 For additive concentrates, weigh into a crucible 1 g of the homogenized blend prepared in 7.6.
8.2 For lubricating oils, weigh into a crucible 3 g of sample or smaller amount estimated to contain not more than 3 g of phosphorus. The amount of sample to be taken is indicated in Table 1.
8.3 Cover the sample with 8 g of zinc oxide and level the surface. Apply heat from a Meker burner to the surface until the zinc oxide becomes red hot; then gently heat the crucible from below with a small bunsen flame so that the oil burns off very gently. Finally, when no more vapor is evolved, ignite strongly and transfer to a muffle furnace at 700°C to burn off residual carbon.
8.4 Allow the crucible to cool and carefully transfer its contents to a 600-mL beaker (Note 3), completing the transfer with a jet of water from a wash bottle. Add about 50 mL of water to the contents of the beaker and rinse the crucible with a few millilitres of concentrated HCl. Add the acid rinsing to the beaker and then sufficient concentrated HCl to bring the total volume of acid added to 23 mL.
8.5 Heat the contents of the beaker until all the ZnO is dissolved, then boil until all hydrogen sulfide has been expelled from the solution (test with lead acetate paper). Allow to cool slightly, add 30 to 50 mg of KBrO3, and boil until all free bromine has been expelled from the solution (test with fluorescein paper).
NOTE 3 - Glass apparatus should have good resistance to alkali. Do not use scratched or etched beakers for the precipitation of quinoline phosphomolybdate.
8.6 Dilute the liquid to a volume of about 150 mL with water, add 30 mL of concentrated HCl and 30 mL of sodium molybdate solution, rinsing the sides of the beaker with a little water after each addition. Place the beaker on the hot plate and bring the liquid to the boil. Add a few drops of quinoline hydrochloride reagent from a coarse-tipped buret or pipet, swirling during the addition.
8.7 Bring to the boil again and add 2 mL of the reagent dropwise with swirling. To the gently boiling liquid add the reagent in 2-mL increments until a total of 24 mL has been added, swirling during the addition. Stand the beaker on the edge of the hot plate or on a boiling water bath for 15 min for the precipitate to settle. Cool to room temperature.
8.8 Prepare a paper pulp pad (Note 4) in a funnel fitted with a porcelain filter disk and tamp down well. Decant the clear supernatant liquid through the filter with applied suction and wash the precipitate twice by decantation with 20-mL portions of 1 M HCl. Transfer the precipitate to the filter pad with cold water and wash the beaker several times with volumes of 25 to 30 mL of water to free the beaker from acid. Use these washes also to wash the precipitate on the filter, allowing each portion of liquid to pass through before pouring on the next. Continue to wash until one portion of wash liquid fails to decolorize the solution when passed into a clean flask containing a few drops of phenolphthalein solution and 1 drop of 0.1 N NaOH solution. Six washes are usually sufficient.
NOTE 4 - This can be made conveniently by placing an intact 20-mm diameter accelerator disk in the funnel, moistening, and applying gentle suction.
8.9 Transfer the precipitate, pad, and disk to the 500-mL conical flask. This can be done conveniently with a glass rod having a drawn-out pointed end. Insert the funnel in the flask and wash into it any portions of the precipitate remaining in the funnel, using CO2-free water. Remove any traces of precipitate adhering to the funnel by wiping with a slip of moist filter-paper; add this to the contents of the flask. Add CO2-free water until the volume of liquid in the flask is about 120 mL.
8.10 Shake the flask until the pulp pad is disintegrated and the precipitate is thoroughly dispersed. Ensure that no lumps remain otherwise difficulty may be encountered in dissolving them in the NaOH solution.
8.11 From a buret slowly add 0.1 M NaOH solution shaking the flask vigorougly to ensure complete solution of the precipitate. Continue to add 0.1 M NaOH solution until the precipitate has dissolved and add approximately 5 mL in excess. Use Table 1 to estimate the volume of alkali required. Record the volume of 0.1 M NaOH solution added (V1). Add about six drops of mixed indicator and titrate with 0.1 M HCl until the color changes from violet through gray and suddenly to pale yellow. Record the volume of 0.1 M HCl used (V2).
8.12 Make a blank determination omitting the sample. In the final titration add 5.0 mL of 0.1 M NaOH solution and titrate with 0.1 M HCl (V3).
9. Calculation
9.1 Blended Samples - Calculate the phosphorus content of blended samples as follows:
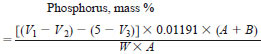
where:
A = mass of original sample in 10 g blend, g
B = mass of white oil in 10-g blend, g,
W = mass of blend taken for determination, g,
V1 = volume of 0.1 M NaOH solution added, mL,
V2 = back titration with 0.1 M HCl, mL,
V3 = back titration, for blank, with 0.1 M HCl, mL.
9.2 Unblended Samples - Calculate the phosphorus content of unblended samples from the following equation:
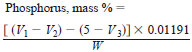
where:
W = weight of sample taken, g,
V1 = volume of 0.1 M NaOH solution added, mL,
V2 = back titration with 0.1 M HCl, mL, and
V3 = back titration, for blank, with 0.1 M HCl, mL.

