ASTM D4045 Standard Test Method for Sulfur in Petroleum Products by Hydrogenolysis and Rateometric Colorimetry
8. Calibration Standard
8.1 Prepare a reference standard-solution or solutions of strength near that expected in the unknown. Measurements can be made by weight or by volume for carrier liquid.
8.2 Units of sulfur in milligrams per litre of sample are preferred as this is independent of the density of the carrier liquid. The following equation is used to calculate the volume of solvent required to dissolve a precise weight of sulfur compound, of known composition and purity to prepare a liquid standard:
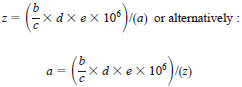
where:
a = desired concentration of sulfur, mg/L, of the standard solution of z millilitre of volume,
b = molecular weight of sulfur: 32.06,
c = molecular weight of the sulfur compound to be used to prepare the standard.
d = mass of the sulfur compound used to prepare the standard, g,
e = purity of sulfur compound expressed as a decimal, and
z = millilitres of standard solution required to give the desired concentration a.
Example:
Calculate the volume of sulfur free isooctane with volume of sulfur compound necessary to dissolve 0.5013 g of 98 % by weight di-n-butyl sulfide to obtain a standard containing 1000 mg/L of sulfur in a solution.
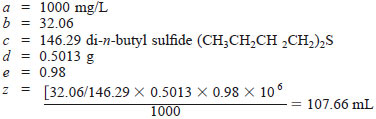
Isooctane is added to bring the solution to a total volume of 107.66 mL. When results are to be reported in mass of parts per million mg/kg, the conversion from milligrams per litre should be done as the last step in the calculations.
8.3 To prepare a sulfur standard with a sulfur concentration of 1000 mg/L, as previously described, obtain a clean 125-mL glass container, a 100-mL flask, and a 20-mL graduated glass pipet. Rinse each thoroughly with 2,2,4-trimethyl pentane (isooctane). (Warning - Extremely flammable.) Pour approximately 90 mL of isooctane into the 100-mL flask. Weigh approximately 0.5 g of di-n-butyl sulfide directly into the flask and record the mass added, to more or less 50 µg. Add additional isooctane to the flask to 100 mL. Transfer the mixture to the 125-mL container and add isooctane equal to the difference of z minus 100 mL. Keep containers closed as much as possible. Do not open containers of pure sulfur compound in the vicinity of sulfur free stocks or low-level standards. Evaporation from containers of pure sulfur compounds can contaminate other nearby liquids. This is particularly troublesome when working below 1 mg/L near volatile sulfur compounds. Volumetrically dilute stock to prepare low-level standards.
9. Calibration and Standardization
9.1 With hydrogen flow at 200 mL/min, advance new tape and note baseline. Adjust the offset up scale about 5 % to be clear of the recorder stops. Record the stable reading average value as the zero sulfur reference and record as R b in 11.1. There will be essentially no difference in reading with or without hydrogen flow and with or without blank injection, if blank and hydrogen have no sulfur.
9.2 Advance the tape and inject a reference material with a sulfur concentration near that expected in the unknown. After about 4 min injection time, adjust the recorder span for approximately 90 % of scale. Record the average reading as Rstd in 10.1.

