12. Procedure
12.1 Place a silicone rubber septum in a bushing and connect to the valve on the sample cylinder containing the gaseous sample (liquefied gas samples are extremely flammable; see A1.7). Crack the cylinder valve so as to flush the air from all connections and then turn the bushing down to hold slight back pressure on the septum. Close the cylinder valve until the gas syringe is ready for filling.
12.2 Crack the valve on the sample cylinder until slight flow of gas is detected around the septum. Insert the gas syringe in the septum carefully. (Warning - High pressure. See A1.8.)
12.3 Withdraw the plunger and allow the gas to flow through the syringe. After sufficient time to flush the syringe with sample, withdraw the plunger so as to contain no less than 5 cm3 of gas.
12.4 Insert the tip of the needle barely through the septum. Inject 5.0 cm3 of gas into the instrument at a constant rate so that 15 s is required for the injection. Determine the sulfur concentration by the procedure described in 10.2-10.7.
12.5 Sulfur concentration can require adjustment of sample volume.
12.6 Report a needle blank with test results.
13. Calculation
13.1 Calculate the sulfur content of the sample in parts per million (ppm) by weight as follows:
Sulfur, mg/kg = (A x F)/W
where:
A = area under curve, taking into account the area of the needle blank, in square centimetres using same range (Ω) as calibration,
W = weight of sample, g, and
F = calibration factor, µg S/cm2
For gases:
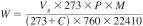
where:
Vg = gas, cm3
P = barometric pressure, mm Hg
M = molecular weight of gas, g/mol, and
C = temperature, gas, °C.
For ethylene at 23°C and 760 mm Hg:
W = Vg x 0.001154
For liquid:
W = VL/1000 x d
where:
VL = volume, µL, and
d = density, g/mL.
13.2 For instruments equipped with a microprocessor or computer, associated instrument software may be used to calculate the sulfur content of the sample in milligrams per kilogram (mg/kg) automatically.

