6. Apparatus
6.1 Pyrolysis Furnace - The sample should be pyrolyzed in an electric furnace having at least two separate and independently controlled temperature zones, the first being an inlet section that can maintain a temperature sufficient to volatilize all the organic sample. The second zone shall be a pyrolysis section that can maintain a temperature sufficient to pyrolyze the organic matrix and oxidize all the organically bound sulfur. A third outlet temperature zone is optional.
6.1.1 Pyrolysis furnace temperature zones for light liquid petroleum hydrocarbons should be variable as follows:

6.2 Pyrolysis Tube, fabricated from quartz and constructed in such a way that a sample, which is vaporized completely in the inlet section, is swept into the pyrolysis zone by an inert gas where it mixes with oxygen and is burned. The inlet end of the tube shall hold a septum for syringe entry of the sample and side arms for the introduction of oxygen and inert gases. The center or pyrolysis section should be of sufficient volume to assure complete pyrolysis of the sample.
6.3 Titration Cell, containing a sensor-reference pair of electrodes to detect changes in triiodide ion concentration and a generator anode-cathode pair of electrodes to maintain constant triiodide ion concentration and an inlet for a gaseous sample from the pyrolysis tube. The sensor electrode shall be platinum foil and the reference electrode platinum wire in saturated triiodide half-cell. The generator anode and cathode half-cell shall also be platinum. The titration cell shall require mixing, which can be accomplished through the use of a magnetic stirring bar, stream of inert gas, or other suitable means. (Warning - Excessive speed will decouple the stirring bar, causing it to rise in the cell and damage the electrodes. The creation of a slight vortex is adequate.)
6.4 Microcoulometer, having variable attenuation gain control, and capable of measuring the potential of the sensing-reference electrode pair, and comparing this potential with a bias potential, amplifying the potential difference, and applying the amplified difference to the working-auxiliary electrode pair so as to generate a titrant. Also the microcoulometer output voltage signal shall be proportional to the generating current.
6.5 Recorder, having a sensitivity of at least 0.1 mV/25 mm with chart speeds of 12 to 25 mm/min. Use of a suitable electronic or mechanical integrator is recommended but optional.
6.6 Sampling Syringe for Liquid - A microlitre syringe of 10-µL capacity capable of accurately delivering 1 to 10 µL of liquid blend into the pyrolysis tube 75 mm by 24-gage needles are recommended to reach the inlet zone of the pyrolysis furnace.
NOTE 3 - Since care should be taken not to overload the pyrolyzing capacity of the tube by too fast a sample injection rate, means should be provided for controlling the sample addition rate (0.1 to 0.2 µL/s).
6.7 Sampling Syringe for Gas - A gas syringe capable of delivering up to 5 cm3 of gas sample into the pyrolysis furnace. A 25-mm by 28-gage needle should be attached to the syringe.
6.8 Exit Tube Insert, with quartz wool.
7. Reagents and Materials
7.1 Purity of Reagents - Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination.
7.2 Purity of Water - The water used in preparing the cell electrolyte should be demineralized or distilled or both. Water of high purity is essential. See Specification D1193 for reagent water.
NOTE 4 - Distilled water obtained from an all borosilicate glass still, fed from a demineralizer, has proven very satisfactory.
7.3 Acetic Acid (rel dens 1.05) - Concentrated acetic acid (CH3COOH). (Warning - May cause burns. See A1.1.)
7.4 Argon, Helium, or Nitrogen , high-purity grade (HP) used as the carrier gas. High-purity grade gas has a minimum purity of 99.995 %. (Warning - Hazardous pressure. See A1.2.)
7.5 Cell Electrolyte Solution - Dissolve 0.5 g of potassium iodide (KI) and 0.6 g ofsodium azide (NaN3) in approximately 500 mL of high-purity water, add 5 mL of acetic acid (CH3COOH) and dilute to 1000 mL.
NOTE 5 - Bulk quantities of the electrolyte should be stored in a dark bottle or in a dark place and be prepared fresh at least every 3 months.
7.6 Gas Regulators - Two-stage gas regulators must be used on the reactant and carrier gas.
7.7 Iodine - (I2), 20 mesh or less, for saturated reference electrode. (Warning - Toxic fumes. See A1.3.)
7.8 Isooctane (2,2,4-trimethyl pentane) - A high purity isooctane of pesticide quality has been found satisfactory. (Warning - Combustible, very harmful. See A1.4.)
NOTE 6 - The most reliable solvent is a sulfur-free form of the sample type to be analyzed. Alternatively, use a high-purity form of cyclohexane [boiling point 80°C (176°F)], isooctane (2,2,4-trimethyl pentane) [boiling point, 99.3°C (211°F)], or hexadecane [boiling point, 287.5°C (549.5°F)].
7.9 n-Butyl Sulfide (CH3CH2CH2CH2)2S.
7.10 Oxygen, high-purity grade (HP), used as the reactant gas. (Warning - Oxygen accelerates combustion. See A1.5.)
7.11 Potassium Iodide (KI), fine granular.
7.12 Sodium Azide (NaN3), fine granular. (Warning - Highly toxic. Can react violently with shock, friction or heat.)
7.13 Sulfur, Standard Solution (approximately 30 mg/kg) - Pipet 10 mL of sulfur stock solution (reagent 7.14) into a 100-mL volumetric flask and dilute to volume with isooctane.
NOTE 7 - The analyst may choose other sulfur compounds for standards appropriate to sample boiling range and sulfur type which cover the concentration range of sulfur expected.
7.14 Sulfur, Standard Stock Solution (approximately 300 ppm (µg/g)) - Weigh accurately 0.5000 g of n-butyl sulfide into a tared 500-mL volumetric flask. Dilute to the mark with isooctane and reweigh.
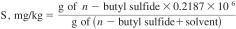
7.15 Calibration Check Sample(s) - portions of one or more liquid petroleum or product standards of known sulfur content and not used in the generation of the calibration curve. A calibration check sample or samples shall be used to verify the validity of the calibration curve as described in Section 10.
7.16 Quality Control (QC) Sample(s) - preferably portions of one or more gaseous petroleum materials that are stable and representative of the samples of interest. These QC samples can be used to verify that the testing process is in statistical control as described in Section 12.

