3. Summary of Test Method
3.1 A sample is injected into a combustion tube maintained at about 800°C having a flowing stream of gas containing about 80 % oxygen and 20 % inert gas (for example, nitrogen, argon, etc.). Oxidative pyrolysis converts the sulfur to sulfur dioxide which then flows into a titration cell where it reacts with triiodide ion present in the electrolyte. The triiodide thus consumed, is coulometrically replaced and the total current required to replace it is a measure of the sulfur present in the sample injected.
3.2 The reaction occurring in the titration cell as sulfur dioxide enters is:
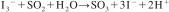
The triiodide ion consumed in the above reaction is generated coulometrically thus:

3.3 These microequivalents of triiodide (iodine) are equal to the number of microequivalents of titratable sample ion entering the titration cell.
3.4 A liquid blend containing a known amount of sulfur is used for calibration.
4. Significance and Use
4.1 Trace quantities of sulfur compounds in hydrocarbon products can be harmful to many catalytic chemical processes in which these products are used. Maximum permissible levels of total sulfur are normally included in specifications for such hydrocarbons. It is recommended that this test method be used to provide a basis for agreement between two laboratories when the determination of sulfur in hydrocarbon gases is important.
4.2 On liquefied petroleum gas, total volatile sulfur is measured on an injected gas sample. For such material a liquid sample must be used to measure total sulfur.
5. Interferences
5.1 This test method is applicable in the presence of total halide concentrations of up to 10 times the sulfur level and total nitrogen content of up to 1.0 %. Free nitrogen does not interfere.
5.2 This test method is not applicable in the presence of total heavy metal concentrations (for example, Ni, V, Pb, etc.) in excess of 500 mg/kg.
NOTE 2 - To attain the quantitative detectability that the method is capable of, stringent techniques should be employed and all possible sources of sulfur contamination must be eliminated.

