ASTM D3120 test method for Trace Quantities of Sulfur in Light Liquid Petroleum Hydrocarbons by Oxidative Microcoulometry
6. Apparatus
6.1 The configuration of the pyrolysis tube and furnace may be constructed as is desirable as long as the operating parameters are met. Fig. 1 is typical of apparatus currently in use.
6.2 A typical assembly and oxidative gas flow through a coulometric apparatus for the determination of trace sulfur is shown in Fig. 2.
6.3 Furnace - Maintained at a temperature sufficient to completely pyrolyze the organic matrix, 900-1200°C, and completely oxidize the organically bound sulfur to SO2. Independently controlled inlet and outlet temperature zones are optional. An electrical furnace has been found suitable to use.
6.4 Pyrolysis Tube - Fabricated from quartz and constructed so the sample is vaporized in a heated zone before the furnace and swept into the oxidation zone by an inert carrier gas, where the vaporized sample mixes with oxygen and is pyrolyzed. The inlet shall be constructed large enough to accommodate a sample boat completely into the oxidation zone of the pyrolysis tube or allow the direct injection of the sample into the heated zone before the furnace. The pyrolysis tube shall have side arms for the introduction of oxygen and inert carrier gas.
6.5 Titration Cell - Consisting of a sensor/reference pair of electrodes to detect changes in triiodide ion concentration, a generator anode-cathode pair of electrodes to maintain a constant triiodide ion concentration, an inlet for gaseous sample from the pyrolysis tube, and an outlet to vent the exit gases from the titration cell. The reference electrode can be either an Ag/AgCl double junction reference electrode or a platinum wire in a saturated triiodide half-cell. The sensor electrode and both the anode and cathode electrodes of the generator are made of platinum. The titration cell shall require mixing, which can be accomplished with a magnetic stir bar, stream of gas, or other suitable means. Other sensor and reference electrodes may be used if they meet the performance criteria of this test method.
NOTE 1 - Take care not to use excessive stirring and possibly damage the electrodes with the stir bar. The creation of a slight vortex is adequate.
6.6 Microcoulometer - The apparatus' microcoulometer, with variable attenuation and gain control, shall be capable of measuring the potential of the sensing-reference electrode pair and compare this potential to a bias potential. By amplifying this potential difference and applying the difference to a working-auxiliary pair of electrodes (the generator), a titrant is generated. The microcoulometer integrates the amount of current used, calculates the equivalent mass of sulfur titrated and calculates the concentration of sulfur in the sample.
6.7 Strip Chart Recorder (Optional) - To monitor and plot the mV potential of the titration cell during the analysis.
6.8 Flow Control - The apparatus shall be equipped with flow controllers capable of maintaining a constant supply of oxygen and inert carrier gas.
6.9 Dryer Tube - The oxidation of samples produces water vapor which, if allowed to condense between the exit of the pyrolysis tube and the titration cell, will absorb the SO2 formed and result in low recovery. Steps shall be taken to prevent such an occurrence. This is easily accomplished by placing a phosphoric acid dehydration tube between the titration cell and exit of the pyrolysis tube. Other approaches, such as heating tape or permeation tubes, can be used if precision and accuracy are not degraded.
6.10 Sampling Syringes - Microlitre syringes able to accurately deliver 5 to 80 mL of sample are required. The volume injected should not exceed 80% of a syringe's capacity.
6.11 Sample Inlet System - Either type of sample inlet system described can be used.
6.11.1 Boat Inlet System - The inlet of the pyrolysis tube is sealed to the boat inlet system. The system provides a cooled area before the furnace for the sample boat prior to quantitative introduction of sample into the boat and is purged with the inert carrier gas. The boat driving mechanism then fully inserts the boat into the oxidation zone of the furnace. The drive mechanism shall advance and retract the sample boat into and out of the oxidation zone of the furnace at a controlled and repeatable rate (see Note 2).
6.11.1.1 Boat Inlet Cooler (Optional) - Sample volatility and injection volume may require an apparatus capable of cooling the sample boat prior to sample introduction. Thermoelectric coolers (peltier) or recirculating refrigerated liquid devices are strongly recommended. Switching sample boats between each analysis may prove effective, provided sample size is not too large.
6.11.1.2 Sample Boats - Quartz or other suitable material which will not react with the sample or sulfur compounds being analyzed and able to withstand the temperatures extremes of the test method.
6.11.2 Syringe Inlet System - The system shall deliver a quantitative amount of sample from a microlitre syringe into a heated area before the oxidation zone of the pyrolysis tube at a controlled and repeatable rate. There the sample is volatilized and the inert carrier gas stream purging the heated area transports the volatilized sample into the oxidation zone of the pyrolysis furnace. An adjustable drive mechanism capable of injecting the sample from a microlitre syringe at a constant rate between 0.5 to 1.0 mL/s is required (see Note 2).
NOTE 2 - Take care not to introduce the sample too fast into the oxidation zone of the furnace and overload the combustion capacity of the pyrolysis tube. Program the sample inlet system to deliver the sample at a sufficiently controlled and repeatable rate to prevent any incomplete combustion by-products (coke or soot) from forming at the exit of the pyrolysis tube.
6.12 Balance - With a weighing precision of +/-0.01 mg.
7. Reagents and Materials
7.1 Purity of Reagents - Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination.
7.2 Purity of Water - Unless otherwise indicated, references to water shall be understood to mean reagent water conforming to Specification D1193, Type II and III.
7.3 Quartz Wool - Grade fine.
7.4 Acetic Acid (CH3COOH) - Glacial acetic acid with specific gravity = 1.05. (Warning - Poison. Corrosive. Combustible. May be fatal if swallowed. Causes severe burns. Harmful if inhaled.)
7.5 Phosphoric Acid (85 % w/w) - Orthophosphoric acid (H3PO4). (Warning - Poison. Corrosive. May be fatal if swallowed. Causes severe burns.)
7.6 Inert Gas - Argon or helium, high purity grade (HP), used as carrier gas. (Warning - Compressed gas under high pressure. Gas reduces oxygen available for breathing.)
7.7 Oxygen - High purity grade (HP), used as the reactant gas. (Warning - Oxygen vigorously accelerates combustion.)
7.8 Gas Regulators - Two-stage gas regulators shall be used for the oxygen and inert carrier gas.
7.9 Cell Electrolyte Solution - Dissolve 0.5 g of potassium iodide (KI) and 0.6 g of sodium azide (NaN3) in approximately 500 mL of high-purity water, add 6 mL of glacial acetic acid (CH3COOH), and dilute to 1000 mL or follow the manufacturer's specifications.
NOTE 3 - Take care to store bulk quantities of the electrolyte in a dark place. It is recommended to prepare fresh electrolyte at least every three months.
7.10 Sodium Azide (NaN3), fine granular. (Warning - Toxic. Causes eye and skin irritation. Explosive.)
7.11 Potassium Iodide (KI), fine granular.
7.12 Potassium Chloride (KCl), fine granular. Used for the 1M Ag/AgCl double junction reference electrode.
7.13 Potassium Nitrate (KNO3), fine granular. Used for the 1M Ag/AgCl double junction reference electrode.
7.14 Iodine (I), 20 mesh or less, for saturated reference electrode.
7.15 Toluene, Xylenes, Isooctane - Reagent grade. (Other solvents similar to those occurring in the samples being analyzed are acceptable.) A solvent blank correction is required due to the inherent sulfur present in the solvents used for standard preparation and sample dilution. (Warning - Flammable solvents. Harmful if inhaled. Vapors may cause flash fire.)
NOTE 4 - The use of solvents with non-detectable levels of sulfur relative to the sulfur content in the sample can make the solvent blank correction unnecessary.
7.16 Dibenzothiophene - FW 184.26, 17.399 % (mass/mass) S (see Note 4).
7.17 n-Butyl Sulfide - FW 146.29, 21.92 % (mass/mass) S (see Note 4).
7.18 Thionaphthene (Benzothiophene) - FW 134.20, 23.90% (mass/mass) S (see Note 4).
NOTE 5 - A correction for chemical impurity can be applied if deemed necessary.
7.19 Sulfur, Standard Solution (approximately 1000 mg-S/mL) - Prepare a stock solution by accurately weighing approximately 0.5748 g of dibenzothiophene or 0.4652 g of n-butyl sulfide or 0.4184 g of thionaphathene into a tared 100 mL, type A volumetric flask. Dilute to volume with a selected solvent. This stock can then be further diluted to prepare sulfur working and calibration standards as outlined in Tables 1-3 (see Notes 6-8).
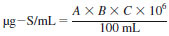
where:
A = grams of standard.
B = weight of fraction sulfur (S) in standard.
C = weight of fraction standard purity.
7.20 Sulfur, Standard Working Solution - Prepare the working standards using standard type A volumetric pipet as outlined in Table 1.
NOTE 6 - Working and calibration standards prepared on a regular basis recommended, depending upon frequency of use and age. Stock solutions typically have a useful life of about 3 months.
NOTE 7 - Calibration standards can be prepared and diluted on a mass/mass basis, when calculation results are adjusted to accommodate them.
NOTE 8 - Stock, working and calibration standards from commercial sources can be used if checked for accuracy and can meet the performance criteria of this test method.
7.21 Quality Assurance (QA) Samples - Samples that are liquid petroleum materials or standards products of known sulfur content that were not used in the generation of the apparatus' calibration curve. These (QA) samples are to be used to check the validity of the testing process as described in Section 11. An ample supply of QA sample material should be available for the intended period of use and shall also be homogenous and stable under the anticipated storage time and conditions.
8. Hazards
8.1 High temperature is employed in this test method. Extra care shall be exercised when using flammable materials near the furnace.
8.2 Consult current OSHA regulations and supplier's Material Safety Data Sheets (MSDS) for materials used in this test method.
9. Sampling
9.1 Obtain a test sample in accordance with Practice D4057 or Practice D4177. To preserve volatile components which are in some samples, do not uncover samples any longer than necessary. Samples should be analyzed as soon as possible after collection from bulk supplies to prevent loss of sulfur, or contamination due to exposure or contact with sample container.
9.1.1 (Warning - Samples collected at temperatures below room temperature can undergo expansion and rupture the container. For such samples, do not fill the container to the top; leave sufficient air space above the sample to allow room for expansion.)
9.2 If the test sample is not used immediately, thoroughly mix the container prior to taking a test specimen.



