ASTM D2896 for base number of petroleum products by potentiometric perchloric acid titration
11. Procedure A (120 mL)
11.1 Calculate the quantity of sample required from its expected base number, BN, as follows:
Approximate weight of sample, g = 28/expected BN
NOTE 10 - For the back titration procedure (see 16.2), or when analyzing used oils, it may be necessary to use a smaller sample weight.
11.1.1 Weigh the sample into the titration beaker, applying the limits shown as follows. A maximum of 20 g should be taken for analysis.
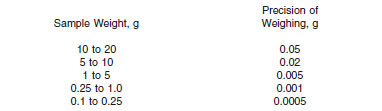
11.2 Add 120 mL of titration solvent to the sample.
11.3 Place the beaker on the titration stand and stir the solution until the sample is dissolved.
NOTE 11 - If solution of the sample proves difficult, dissolve it in 80 mL of chlorobenzene in the titration beaker, then add 40 mL of glacial acetic acid. Many used oils contain some solid materials that will not dissolve. This is a frequently observed condition.
11.4 Prepare the electrodes as directed in 10.1, 10.2, and 10.3. Position the electrodes in the solution so that they are immersed as far as possible. Continue stirring throughout the determination at a rate sufficient to produce vigorous agitation without spattering and without stirring air into the solution. Adjust the meter so that it reads in the upper part of the millivolt scale; for example, 700 mV. For simple meters without this adjustment, it is necessary to incorporate a source of potential in series with the electrode. A 1.5-V dry cell and potential divider is suitable.
11.5 Fill the buret with 0.1 N HClO4 solution and place the buret in position in the titration assembly, taking care that the tip is immersed below the level of the surface of the liquid in the beaker. Record the initial buret and meter (cell potential) readings.
11.6 Titration:
11.6.1 Manual Titration - Add suitable small portions of titrant and, after waiting until a constant potential has been established (Note 12), record the buret and meter readings. At the start of the titration and in any subsequent regions (inflections) where 0.1 mL of titrant consistently produces a total change of more than 0.03 V (corresponding to 0.5 pH scale unit) in the cell potential, add 0.05-mL portions. In the intermediate regions (plateaus) where 0.1 mL increments change the potential by less than 0.03 V, add large portions sufficient to produce a total potential change approximately equal to, but not greater than, 0.03 V. Titrate in this manner until the potential changes less than 0.005 V (corresponding to 0.1 pH scale unit) per 0.1 mL.
NOTE 12 - Consider the cell potential constant when it changes less than 0.005 V/min.
11.6.2 Automatic Recording Titration - Adjust the instrument in accordance with the manufacturer's instructions and set the titration speed at 1.0 mL/min maximum.
11.7 On completion of the titration, remove the beaker and rinse the electrodes and buret tip with titration solvent, then with water, then again with titration solvent (see 10.3). Store in water when not in use (see 10.1).
11.8 For each set of samples make a blank titration using 120 mL of titration solvent. For a manual titration add 0.1 N HClO4 solution in 0.05-mL increments, waiting between each addition until a constant cell potential is established. Record meter and buret readings after each increment. Follow the procedure in 11.6.2 for an automatic titration.

