8. Calculation and Report
8.1 Calculate the hydroxyl value as the number of milligrams of potassium hydroxide equivalent to the hydroxyl content of 1 g of sample as follows:
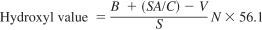
where:
A = KOH solution required for titration of the acid value, mL,
B = KOH solution required for titration of the reagent blank, mL,
C = sample used for the acid value, g,
V = KOH solution required for titration of the acetylated specimen, mL, and
S = sample used for acetylation, g.
8.2 Report the results to the first decimal place.
NOTE 1 - For routine analysis, the ethanol acid value may be substituted in most cases for the pyridine acid value, and the calculation altered accordingly.
9. Precision and Bias
9.1 The following criteria should be used for judging the acceptability of results at the 95 % confidence level:
9.1.1 Repeatability - Duplicate results by the same operator should be considered suspect if they differ by more than 2.4.
9.1.2 Reproducibility - Two results, each the mean of duplicate determinations, obtained by operators in different laboratories should be considered suspect if they differ by more than 3.0.
9.2 Bias - Bias has not been determined.
10. Keywords
10.1 drying oils; hydroxyl value; fatty acids; hydroxyl value

