ASTM D1533 standard test method water in insulating liquids by Coulometric Karl Fischer Titration
1. Scope
1.1 This test method covers the measurement of water present in insulating liquids by coulometric Karl Fischer titration. This test method is used commonly for test specimens below 100 % relative saturation of water in oil. The coulometric test method is known for its high degree of sensitivity (typically 10 µg H2O). This test method requires the use of equipment specifically designed for coulometric titration.
1.2 This test method recommends the use of commercially available coulometric Karl Fischer titrators and reagents.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practice and determine the applicability of regulatory limitations prior to use. For specific precautionary statements see 8.1 and A2.1.
2. Referenced Documents
2.1 ASTM Standards:
D923 Practices for Sampling Electrical Insulating Liquids
2.2 IEC Standard:
IEC 60814: Insulating Liquids - Oil-Impregnated Paper and Pressboard - Determination of Water by Automatic Coulometric Karl Fischer Titration
3. Summary of Test Method
3.1 This test method is based on the reduction of iodine containing reagent according to the traditional Karl Fischer reaction. The proposed reaction mechanism is as follows:
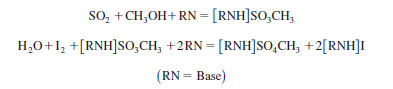
The endpoint is determined amperometrically with a platinum electrode that senses a sharp change in cell resistance when the iodine has reacted with all of the water in the test specimen.
3.2 The coulometric Karl Fischer test method requires the use of an automatic titrator with commercially available reagents. Karl Fischer instruments regenerate iodine coulometrically from the iodide in the Karl Fischer reagent. The test specimen is injected into a titration cell where the iodine consumed by the reaction with water is electrolytically regenerated by anodic oxidation of iodide. The completion of the reaction is detected with a platinum sensing electrode. The coulombs of electricity required to generate the necessary amount of iodine then is converted into the amount of water present in the test specimen by use of the Faraday equation.
3.3 Titration Cell - The coulometric titration cell consists of either a sealed vessel containing both an anode and cathode which are separated by a diaphragm or a sealed vessel containing an anode and cathode which are not separated by a diaphragm. In both cells the anode compartment contains a solution consisting of sulfur dioxide, iodide, and an amine in a solvent containing methanol/chloroform or methanol/longer chain alcohol. In the cell with a diaphragm the cathode compartment contains similar reagents optimized for cathodic reduction.

