ASTM D117 standard guide for sampling, test methods and specifications for electrical insulating oils of petroleum origin
CHEMICAL PROPERTIES
22. Acidity, Approximate
22.1 Scope - Test Method D1534 covers the determination of the approximate total acid value of used electrical insulating liquids, in general, those having viscosities less than 24 cSt at 40°C. It is a simple procedure that can be applied in the field.
22.2 Summary of Test Method:
22.2.1 Test Method D1534 - To determine whether the acidity is greater or less than a fixed arbitrary value, a fixed volume of liquid to be tested is added to the test bottle or graduated cylinder, together with a small amount of indicator (phenolphthalein) and the appropriate quantity of standard potassium hydroxide solution. The mixture is shaken and allowed to separate. The color of the aqueous layer at the bottom of the container when testing mineral oils, or at the top when testing askarels, determines whether the acidity is less than or greater than the arbitrary value chosen.
22.3 Significance and Use:
22.3.1 The approximate acidity of used electrical insulating oils is an estimate of the total acid value of the oil. As acid values increase, usually due to oxidation of the oil in service, the impairment of those oil qualities, important to proper functioning of specific apparatus, increases. In general, acidic by-products produce increased dielectric loss, increased corrosivity, and may cause thermal difficulties attributable to insoluble components called "sludge". This test method is adapted to a specific volume of oil; total acid values of 0.05 to 0.5 mg of potassium hydroxide per gram of oil is a range which is functionally significant.
23. Carbon-Type Composition
23.1 Scope - This test method covers the determination of carbon-type composition of insulating oils by correlation with basic physical properties. Carbon-type composition is expressed as percentage of aromatic carbons, percentage of naphthenic carbons, and percentage of paraffinic carbons. Viscosity, relative density (or specific gravity), and refractive index are the only measurements required for use of this test method.
23.2 Summary of Test Method:
23.2.1 Test Method D2140 - The viscosity, density and specific gravity, and refractive index of the oil are measured. From these values, the viscosity-gravity constant and refractivity intercept are calculated. Using these two computed values, percentage of aromatic carbons, naphthenic carbons, and paraffinic carbons are estimated from a correlation chart.
23.3 Significance and Use - The primary purpose of this test method is to characterize the carbon-type composition of an oil. It is also applicable in observing the effect on oil constitution of various refining processes, such as solvent extraction, acid treatment, and so forth. It has secondary application in relating the chemical nature of an oil to other phenomena that have been demonstrated to be related to oil composition.
24. Compatibility with Construction Material
24.1 Scope - This test method covers screening for the compatibility of materials of construction with electrical insulating oil for use in electrical equipment. Solid materials that can be tested for compatibility include varnishes, dip coatings, core steel, core steel coatings, gaskets, and wire enamels.
24.2 Summary of Test Method:
24.2.1 Test Methods D3455 - The electrical insulating oil and the material whose compatibility is being tested are aged for 164 h at 100°C. Changes in the oil and compatibility sample are observed and appropriate tests conducted.
24.3 Significance and Use:
24.3.1 The magnitude of the change in the electrical properties of the insulating oil is of importance in determining the contamination of the oil by the test specimen.
24.3.2 Physical and chemical changes in the oil such as color, interfacial tension, and acidity also indicate solubility or other adverse effects of the test specimen on the oil.
24.3.3 The physical changes of the test specimen, such as hardness, swelling, and discoloration, show the effect of the oil on the test specimen and are used to determine the suitability of the material for use in insulating oil.
24.3.4 A material meeting the criteria recommended does not necessarily indicate suitability for use in electrical equipment. Other properties must also be considered. Additionally, certain materials containing additives may meet the requirements of this procedure, yet be unsatisfactory when subjected to longer term evaluations.
25. Copper Content
25.1 Scope:
25.1.1 Test Method D3635 - Covers the determination of copper in new or used electrical insulating oil. For flame atomization, the lower limit of detectability is of the order of 0.1 ppm. For nonflame atomization, the lower limit of detectability is less than 0.01 ppm.
25.2 Summary of Test Method:
25.2.1 Test Method D3635 - The test specimen of oil is diluted with an appropriate organic solvent and analyzed in an atomic absorption spectrophotometer. Alternative procedures are provided for instruments employing flame and nonflame atomization. Concentration is determined by means of calibration curves prepared from standard samples.
25.3 Significance and Use - Electrical insulating oil may contain small amounts of dissolved metals derived either directly from the base oil or from contact with metals during refining or service. When copper is present, it acts as a catalyst in promoting oxidation of the oil. This test method is useful for research and to assess the condition of service-aged oils.
26. Furanic Compounds
26.1 Scope - Test Method D5837 covers the determination, in electrical insulating liquids, of the products of the degradation of cellulosic materials such as paper, pressboard, and cotton material typically found as insulating materials in electrical equipment. These degradation products are substituted furan derivatives, commonly referred to as furanic compounds or furans.
26.1.1 The commonly identified furans that may be identified by this method include: 5-hydroxymethyl-2-furaldehyde, furfuryl alcohol, 2-furaldehyde, 2-acetylfuran, and 5-methyl-2-furaldehyde.
26.2 Summary of Test Method - Furanic compounds in electrical insulating liquids are extracted from a known volume of test specimen by means of a liquid/liquid extraction or solid-phase extraction. A method for direct introduction of oil into the chromatograph is also described. An aliquot of the extract is introduced into a High Performance Liquid Chromatography (HPLC) system equipped with a suitable analytical column and UV detector. Furanic compounds in the test specimen are identified and quantified by comparison to standards of known concentration.
26.3 Significance and Use - Furanic compounds are generated by the degradation of cellulosic materials used in solid insulation systems of electrical equipment. Furanic compounds which are oil soluble to an appreciable degree will migrate into the insulating liquid. High concentrations or unusual increases in the concentration of furanic compounds in oil may indicate cellulose degradation from aging or incipient fault conditions.
27. Gas Analysis
27.1 Scope:
27.1.1 This test method covers three procedures for the extraction and measurement of gases dissolved in electrical insulating oil having a viscosity of 20 cSt (100 Saybolt Universal seconds) or less at 40°C (104°F), and the identification and determination of the individual component gases extracted.
27.1.2 The individual component gases that may be identified and determined include: hydrogen, oxygen, nitrogen, carbon monoxide, carbon dioxide, methane, ethane, ethylene, acetylene, propane, and propylene.
27.2 Summary of Test Methods:
27.2.1 Method A (Test Method D3612) - Dissolved gases are extracted from a sample of oil by introduction of the oil sample into a pre-evacuated known volume. The evolved gases are compressed to atmospheric pressure and the total volume measured.
27.2.2 Method B (Test Method D3612) - Dissolved gases are extracted from a sample of oil by sparging the oil with the carrier gas on a stripper column containing a high surface area bead.
27.2.3 Method C (Test Method D3612) - The sample is brought in contact with a gas phase in a sealed vial and the dissolved gases are allowed to equilibrate with the gas phase. The headspace above the oil is sampled and analyzed. The amount of dissolved gasses in the oil is calculated from predetermined partition coefficients for each gas.
27.2.4 There may be some differences in limits of detection and precision and bias between Methods A, B, and C for the various gases.
27.2.5 A portion of the extracted gases (Methods A and C) or all of the gases extracted (Method B) are introduced into a gas chromatograph equipped with suitable adsorption column(s). The composition of the sample is calculated from its chromatogram by comparing the area of the peak of each component with the area of the peak of the same component on a reference chromatogram made on a standard mixture of known composition.
27.3 Significance and Use:
27.3.1 Oil and oil-immersed electrical insulating materials may decompose under the influence of thermal and electrical stresses and in doing so generate gaseous decomposition products of varying composition, which dissolve in the oil. The nature and amount of the individual component gases that may be recovered and analyzed may be indicative of the type and degree of the abnormality responsible for the gas generation. The rate of gas generation and changes in concentration of specific gases over time are also used to evaluate the condition of the electric apparatus.
28. Gas Content
28.1 Scope:
28.1.1 Test Method D831 - Electrical insulating oils of low and medium viscosities up to 190 cSt at 40°C (corresponding to 1000 SUS at 100°F), including oils used in capacitors and paper-insulated electric cables and cable systems of the oil-filled type.
28.1.2 Test Method D1827 - Electrical insulating liquids with a viscosity of 216 cSt (1000 SUS) or less at 100°C. Acidic gases absorbed by a strong caustic solution are not detectable. Carbon dioxide and hydrogen chloride will not be included in gas content determined by this test method.
28.1.3 Test Method D2945 - Electrical insulating oils of up to 19 cSt at 40°C (corresponding to 100 SUS at 100°F). This test method is suitable for either field or laboratory use. It was designed to use a self-contained apparatus in the analysis of oils of low gas content. Unlike Test Methods D831 and D1827, gas content is not corrected for ambient temperature or pressure.
28.2 Definition:
28.2.1 gas content of an oil by volume - The total volume of gases, corrected to 101 kPa (760 mm Hg) and 0°C, contained in a given volume of oil, expressed as a percentage.
28.3 Summary of Test Methods:
28.3.1 Test Method D831 - The oil is fed slowly into a degassing chamber, located in an oven and initially evacuated to a pressure below 13 Pa (0.1 Torr) with a vacuum pump, so that the oil is thoroughly exposed to the vacuum. Condensable gases are removed from the system by a cold trap. The gas volume is calculated from the increase in pressure in the degassing chamber, measured by a McLeod gage.
28.3.2 Test Method D1827 - A small liquid sample is purged of dissolved gases with pure carbon dioxide gas. The gas stream is then led into a gas burette containing a potassium hydroxide solution. The carbon dioxide and any other acidic gases are completely absorbed, and the volume of the remaining gas is measured.
28.3.3 Test Method D2945 - The oil sample is allowed to flow as a thin film into a chamber evacuated by the lowering of a connecting mercury reservoir. By raising the mercury reservoir, the pressure is returned to atmospheric, and the volume of the evolved gases is measured. No correction is made for atmospheric pressure or ambient temperature.
28.4 Significance and Use:
28.4.1 Some types of electrical equipment require use of electrical insulating liquids of low gas content. Capacitors and certain types of electrical cable, particularly where used at high voltages, may suffer from the formation of gas bubbles with consequent gaseous ionization if gas content is not sufficiently reduced. In filling electrical apparatus, a low gas content reduces foaming and also reduces available oxygen in sealed equipment, increasing the service life of the insulating oil.
28.4.2 These tests are not intended for use in purchase specifications because the oil is customarily degassed immediately before use. These test methods can be used, however, as a factory control test and a control and functional test in installation and maintenance work by utilities. These tests require care in manipulation and the availability of trained, careful personnel.
28.5 Precision:
28.5.1 The precisions of two of the test methods are given in the table below. Refer to the original test methods for the conditions under which these precision values are applicable.
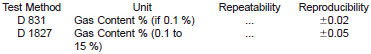
29. Inorganic Chlorides and Sulfates
29.1 Scope - This test method covers the qualitative determination of inorganic chlorides and sulfates in electrical insulating oils.
29.2 Summary of Test Method:
29.2.1 Test Method D878 - The electrical insulating oil is extracted with water. The water layer is tested with silver nitrate solution for the presence of chlorides and with barium chloride for the presence of sulfates. If a precipitate is obtained with either reagent, report the corresponding ion as present.
29.3 Significance and Use - The presence of inorganic chlorides or sulfates may be due either to improper refining or to contamination from outside sources. The presence of these contaminants may affect the corrosivity or dielectric properties of the oil and may adversely affect its ability to function properly under service conditions.
30. Neutralization Number
30.1 Scope:
30.1.1 The two procedures available determine the acidic or basic constituents in petroleum products. Because the titration end points of these methods differ, results may differ between the test methods.
30.1.2 Test Method D664 - Resolves the constituents into weak-acid and strong-acid components, provided the dissociation constants of the more highly ionized compounds are at least 1000 times that of the next weaker group. Because the end point is determined potentiometrically, this test method is suitable for use with very dark samples.
30.1.3 Test Method D974 - Applicable for the determination of acids or bases whose dissociation constants in water are larger than 10(-9). Constituents are classified as strong acid, weak acid, or strong base. Excessively dark-colored oils cannot be tested by this test method due to obscuration of the color indicator end point.
30.2 Definitions:
30.2.1 total acid number - the number of milligrams of KOH required to neutralize all acidic constituents present in 1 g of test specimen. When neutralization number is specified without further qualification, total acid number is implied.
30.2.2 strong acid number - the number of milligrams of KOH required to neutralize the strong acid constituents present in 1 g of test specimen.
30.3 Summary of Test Methods:
30.3.1 Test Method D664 - The test specimen is dissolved in a mixture of toluene and isopropyl alcohol containing a small amount of water and titrated potentiometrically with alcoholic potassium hydroxide or hydrochloric acid solution, using a glass-indicating electrode and a calomel reference electrode. The meter readings are plotted against the respective volumes of titrating solution, and the end points are taken at the inflections in the resulting curve. When no definite inflections are obtained, end points are taken at meter readings corresponding to those found for standard nonaqueous acidic and basic buffer solutions.
30.3.2 Test Method D974 - To determine the total acid or strong base number, the test specimen is dissolved in a mixture of toluene and isopropyl alcohol containing a small amount of water, and the resulting single-phase solution is titrated at room temperature with standard alcoholic base or alcoholic acid solution, respectively, to the end point indicated by the color change of the added p-naphtholbenzein solution. To determine the strong acid number, a separate portion of the sample is extracted with hot water, and the aqueous extract is titrated with potassium hydroxide solution, using methyl orange as an indicator.
30.3.3 Modified Test Method D974 - For acid numbers less than 0.04, use of a closed microburet (5 mL, 0.02-mL subdivisions) with preneutralized titration solvent, shielding the titration flask with a rubber cap through which the buret tip extends, is recommended.
30.3.3.1 For service-aged test specimens from electrical apparatus in which insulation deterioration could result in the solution of carbon dioxide in electrical insulating oil, the test specimen may be freed of carbon dioxide by blowing for 2 min at room temperature with nitrogen prior to testing.
30.3.3.2 When using the modification of Test Method D974 as noted in 28.3.3, the following precision applies:
30.3.3.3 Repeatability - Duplicate determinations by the same operator should not differ by more than 0.008. For two operators in the same laboratory, each making duplicate determinations and comparing average values, results should not differ by more than 0.005 (95 % probability).
30.3.3.4 Reproducibility - Results from two different laboratories, comparing average values from duplicate determinations, should not differ by more than 0.015 (95 % probability).
30.4 Significance and Use:
30.4.1 A low total acid content of an insulating oil is necessary to minimize electrical conduction and metal corrosion and to maximize the life of the insulation system.
30.4.2 In used insulating oils, an increase in total acid number from the value of the unused product indicates contamination by substances with which the oil has been in contact or a chemical change in the oil from processes such as oxidation. An increase in total acid number may indicate the desirability of replacing used with fresh oil, provided suitable rejection limits have been established and other tests confirm the need for the change.
31. Oxidation Inhibitor Content
31.1 Scope:
31.1.1 New electrical insulating oil may contain inhibitors to inhibit oxidation. Two test methods are available for the determination of the commonly used inhibitors.
31.1.2 Test Method D2668 - Determines the concentration of either inhibitor, or their mixtures, in concentrations up to 0.5 mass %, by measuring the infrared absorbance of the oil at selected frequencies.
31.1.3 Test Method D4768 - This test measures the concentration of either inhibitors or their mixtures, in concentrations up to 0.5 % mass, by gas chromatographic separation and quantitation to a suitable standard.
31.2 Summary of Test Methods:
31.2.1 Test Method D2688 - The infrared absorbance of the test specimen is measured at the frequencies appropriate to 2,6-ditertiary-butyl para-cresol and 2,6-ditertiary-butyl phenol and the concentrations calculated from a calibration curve.
31.2.2 Test Method D4768 - A column clean-up is employed to remove interfering substances, followed by a gas chromatographic separation and concentration measured by comparison to suitable standards.
31.3 Significance and Use - The quantitative determination of 2,6-ditertiary-butyl para-cresol or 2,6-ditertiary-butyl phenol measures the amount of this material that has been added to new electrical insulating oil as protection against oxidation or the amount remaining in a used oil. These test methods are also suitable for manufacturing control and for use as specification acceptance tests.
32. Oxidation Stability
32.1 Scope:
32.1.1 Three oxidation test methods are applied to insulating oil:
32.1.2 Test Method D1934 - Covers two procedures for subjecting electrical insulating oils to oxidative aging: Procedure A, without a metal catalyst, and Procedure B, with a metal catalyst.
32.1.2.1 This test method is applicable to oils used as impregnating or pressure media in electrical power transmission cables as long as less than 10 % of the oil evaporates during the aging procedures. It applies and is generally useful primarily in the evaluation and quality control of unused oils, either inhibited or uninhibited.
32.1.2.2 The precision statement for Test Method D1934 should be the standard deviation of the logarithm of the dissipation factor ratios rather than the coefficient of the variation of the ratios.
32.1.3 Test Method D2112 - Is intended as a rapid method for the evaluation of the oxidation stability of new mineral insulating oils containing oxidation inhibitor. This test is considered of value in checking the oxidation stability of new mineral insulating oils containing synthetic oxidation inhibitor in order to control the continuity of this property from shipment to shipment. The applicability of this procedure for use with inhibited insulating oils of more than 12 cSt at 40°C has not been established.
32.1.4 Test Method D2440 - Covers the evaluation of the acid- and sludge-forming tendency of new mineral transformer oils. It is considered of value in studying the acid- and sludge-forming propensity of a new grade of mineral transformer oil before commercial application.
32.2 Summary of Test Methods:
32.2.1 Test Method D1934 - This test method consists of exposing for 96 h 300 mL of oil in a 400-mL beaker to moving air in an oven controlled at 115°C, with or without 15 cm2 of metal catalyst. Changes in such properties as color, total acid number, power factor, and resistivity of the aged oil can be used to determine the oxidative deterioration of the oil.
32.2.2 Test Method D2112 - The test specimen is agitated by rotating axially at 100 rpm at an angle of 30° from the horizontal under an initial oxygen pressure of 620 kPa (90 psi) in a pressure vessel with a glass sample container and copper catalyst coil, in the presence of water, at a bath temperature of 140°C. The time for an oil to react with a given volume of oxygen is measured; completion of the test is indicated by a 172 kPa (25 psi) drop in pressure.
32.2.3 Test Method D2440 - The test oil is charged to a glass oxidation tube containing copper wire catalyst. The tube is placed in an oil bath at 110°C, and oxygen is bubbled through separate oil samples for 72 and 164 h. The n-heptane insoluble sludge and total acid number of the aged oil is measured to determine the extent of oxidation.
32.3 Significance and Use:
32.3.1 The development of oil sludge and acidity resulting from oxidation during storage, processing, and long service life should be held to a minimum. This minimizes electrical conduction and metal corrosion, maximizes insulation system life and electrical breakdown strength, and ensures satisfactory heat transfer.
32.3.2 The oxidation stability tests described in Section 32 may be used to evaluate the tendency to form sludge or acids under oxidizing conditions, to ensure the continuity of quality of mineral insulating oil shipments, and for specification purposes. A low tendency to form sludge and acid in laboratory tests is desirable, although the oil showing the least deterioration in the laboratory is not necessarily the best in service.
32.3.3 The oxidation stability tests are used in the following specifications for insulating oils:

33. Polychlorinated Biphenyl Content
33.1 Scope:
33.1.1 Test Method D4059 - Describes a quantitative technique for determining the concentration of polychlorinated biphenyls (PCBs) in electrical insulating liquids by gas chromatography.
33.2 Definition:
33.2.1 PCB concentration - is normally expressed in units of parts per million (PPM) on a weight by weight basis. Standard chromatograms of Aroclors 1242, 1254, and 1260 are used to determine the concentration of PCB in the sample.
33.3 Summary of Test Method - Following dilution of the test specimen in a suitable solvent, the solution is treated to remove interfering substances. A small portion is then injected into a gas chromatographic column where the components are separated and their presence measured by an electron capture or halogen-specific electrolytic conductivity detection. The test method is made quantitative by comparing the response of a sample to that of a known quantity of one or more standard Aroclors obtained under the same conditions.
33.4 Significance and Use - United States' regulations require that electrical apparatus and electrical insulating fluids containing PCB be handled and disposed of through the use of specific procedures as determined by the PCB content of the fluid. The results of this test method can be useful in selecting appropriate handling and disposal procedures.
34. Sediment and Soluble Sludge
34.1 Scope - This test method covers the determination of sediment and soluble sludge in service-aged insulating oils of petroleum origin. Also, provision is made for determining organic and inorganic content of the sediment. The test method is intended primarily for oils of comparatively low viscosity, for example, 7 to 15 cSt at 40°C. Suitability for high-viscosity oils has not been determined.
34.2 Summary of Test Method:
34.2.1 Test Method D1698 - A test specimen portion is centrifuged to separate sediment from the oil. The upper, sediment-free portion is decanted and retained for determination of soluble sludge. The sediment is dislodged and filtered through a specially prepared Gooch crucible. After drying and weighing to obtain total sediment, the crucible is ignited at 500°C and reweighed. Loss in weight is organic and remainder is inorganic content of sediment. Soluble sludge is determined on sediment-free portion by dilution with n-pentane to precipitate n-pentane insolubles, and filtration through a Gooch crucible.
34.3 Significance and Use:
34.3.1 Sediment in insulating oil may deposit on transformer parts and interfere with heat transfer, and may choke oil ducts, and so hinder oil circulation and heat dissipation. Inorganic sediment usually indicates contamination of some type, and organic sediment indicates either deterioration of the oil or contamination.
34.3.2 Soluble sludge indicates deterioration of the oil, presence of contaminants, or both. It serves as a warning that formation of sediment may be imminent.
34.3.3 The determination of sediment and soluble sludge in a used insulating oil assists in deciding whether the oil may continue to be used in its existing condition or should be replaced, reclaimed, or reconditioned.
35. Sulfur, Corrosive
35.1 Scope - This test method covers the detection of corrosive sulfur compounds in electrical insulating oils of petroleum origin. Compounds capable of severely discoloring a copper surface under prescribed test conditions are reported as corrosive.
35.2 Summary of Test Method:
35.2.1 Test Method D1275 - A polished copper specimen is exposed to the test oil for 19 h at 140°C. The appearance of the copper surface determines whether the oil is classified as corrosive or noncorrosive.
35.3 Significance and Use - In most of their uses, insulating oils are continually in contact with metals that are subject to corrosion, such as copper or silver. Since the presence of detrimental corrosive sulfur compounds will result in deterioration of these metals to an extent dependent upon the quantity and type of corrosive agent and the time and temperature factors, the detection of these undesirable impurities, even though not in terms of quantitative values, is a means for recognizing the hazard involved.
36. Water Content
36.1 Scope - Test Method D1533 covers the determination of water present in insulating liquids, in concentrations most commonly below 200 ppm.
36.2 Summary of Test Method:
36.2.1 This test method is based on the reduction of iodine in accordance with the traditional Karl Fischer reaction.
36.2.2 Test Method D1533 electrochemically generates the iodine required for Karl Fischer titration.
36.2.3 This automatic coulometric titration procedure requires the use of an instrument that is designed and calibrated to deliver a known electrical current which generates sufficient iodine to neutralize a known weight of water per minute. The two-part titration solution is first brought to near a zero dryness by iodine produced by the generator when the controls are placed in the "standby" setting. The test specimen is added; and the titration begun, allowing the test specimen to be automatically titrated by producing iodine at the generator anode until the equivalent point is reached and the titration is complete. Water content is read directly on the meter in micrograms (or parts per million).
36.3 Significance and Use - A low water content of insulating oil is necessary to achieve adequate electrical strength and low dielectric loss characteristics, to maximize the insulation system life, and to minimize metal corrosion. Water in solution cannot be detected visually and must be determined by other means. This test shows the presence of water that may not be evident from electrical tests.

