ASTM D1159 Standard Test Method for Bromine Numbers of Petroleum Distillates and Commercial Aliphatic Olefins by Electrometric Titration
9. Procedure
9.1 Place 10 mL of 1,1,1-trichloroethane or dichloromethane in a 50-mL volumetric flask and, by means of a pipet, introduce a test specimen as indicated in Table 2. Either obtain the weight of specimen introduced by difference between the weight (to the nearest 1 mg) of the flask before and after addition of specimen or, if the density is known accurately, calculate the weight from the measured volume. Fill the flask to the mark with the selected solvent and mix well. (Warning - Hydrocarbons, particularly those boiling below 205°C (400°F), are flammable.)
9.1.1 Frequently, the order of magnitude of the bromine number of a specimen is unknown. In this case, a trial test is recommended using a 2-g specimen in order to obtain the approximate magnitude of the bromine number. This exploratory test shall be followed with another determination using the appropriate specimen size as indicated in Table 2.
9.1.2 The test specimen taken shall not exceed 10 mL and the volume of bromide-bromate titrant used shall not exceed 10 mL and no separation of the reaction mixture into two phases shall occur during the titration. Difficulty may be experienced in dissolving specimen of the high boiling ranges in the titration solvent; this can be prevented by the addition of a small quantity of toluene.
9.2 Cool the titration vessel to 0 to 5°C (32 to 41°F) and maintain the contents at this temperature throughout the titration. Switch on the titrimeter, and allow the electrical circuit to become stabilized.
9.3 Introduce 110 mL of titration solvent into the vessel and pipet in a 5-mL aliquot of the sample solution from the 50-mL volumetric flask. Switch on the stirrer and adjust to a rapid stirring rate, but avoid any tendency for air bubbles to be drawn down to the solution.
9.4 Set the end point potential. With each instrument, the manufacturer's instructions should be followed for end point setting and to achieve the sensitivity in the platinum electrode circuit specified in 6.1.
9.5 Depending on the titrator apparatus, add the bromide-bromate solution manually or by microprocessor control in small increments from the buret. The endpoint of the titration is achieved when the potential reaches the pre-set value (see 9.4) and persists for more than 30 s.
9.6 Blanks - Perform duplicate blank titrations of each batch of titration solvent. Do this by repeating 9.3 through 9.5 for each blank determination, substituting 5 mL of the selected solvent (1,1,1-trichloroethane or dichloromethane) in place of the sample solution. Less than 0.1 mL of bromide-bromate solution should be required. If more than 0.1 mL is used, discard the analysis, prepare fresh titration solvent and fresh reagents and repeat the analysis.
10. Calculation
10.1 Calculate the bromine number as follows:
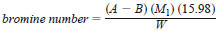
where:
A = millilitres of bromide-bromate solution required for titration of the test aliquot,
B = millilitres of bromide-bromate solution required for titration of the blank,
M1 = molarity of the bromide-bromate solution, as Br2,
W = grams of test specimen in the aliquot, and
15.98 = factor for converting g of bromine per 100 g of specimen and incorporating molecular weight of bromine (as Br 2) and conversion of mL to L.

