ASTM D1159 Standard Test Method for Bromine Numbers of Petroleum Distillates and Commercial Aliphatic Olefins by Electrometric Titration
6. Apparatus
6.1 Electrometric End Point Titration Apparatus - Any apparatus designed to perform titrations to pre-set end points (see Note 2) may be used in conjunction with a high-resistance polarizing current supply capable of maintaining approximately 0.8 V across two platinum electrodes and with a sensitivity such that a voltage change of approximately 50 mV at these electrodes is sufficient to indicate the end point. Other types of commercially available electronic titrimeters, including certain pH meters, have also been found suitable.
NOTE 2 - Pre-set end point indicated with polarized electrodes provides a detection technique similar to the dead stop technique specified in previous versions of this test method.
6.2 Titration Vessel - A jacketed glass vessel approximately 120 mm high and 45 mm in internal diameter and of a form that can be conveniently maintained at 0 to 5°C (32 to 41°F).
6.3 Stirrer - Any magnetic stirrer system.
6.4 Electrodes - A platinum wire electrode pair with each wire approximately 12 mm long and 1 mm in diameter. The wires shall be located 5 mm apart and approximately 55 mm below the level of the titration solvent. Clean the electrode pair at regular intervals with 65 % nitric acid and rinse with distilled water before use.
6.5 Buret - Any delivery system capable of measuring titrant in 0.05 mL or smaller graduations.
7. Reagents
7.1 Purity of Reagents - Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all reagents shall conform to the specifications of the committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination.
7.2 Purity of Water - Unless otherwise indicated, references to water shall be understood to mean reagent water as defined by Type III of Specification D1193.
7.3 Acetic Acid, Glacial - (Warning - Poison, corrosive-combustible, may be fatal if swallowed. Causes severe burns, harmful if inhaled.)
7.4 Bromide-Bromate, Standard Solution (0.2500 M as Br2) - Dissolve 51.0 g of potassium bromide (KBr) and 13.92 g of potassium bromate (KBrO3) each dried at 105°C (220°F) for 30 min in water and dilute to 1 L.
7.4.1 If the determinations of the bromine number of the reference olefins specified in Section 8 using this solution do not conform to the prescribed limits, or if for reasons of uncertainties in the quality of primary reagents it is considered desirable to determine the molarity of the solution, the solution shall be standardized and the determined molarity used in subsequent calculations. The standardization procedure shall be as follows:
7.4.1.1 To standardize, place 50 mL of glacial acetic acid and 1 mL of concentrated hydrochloric acid (Warning - Poison corrosive. May be fatal if swallowed. Liquid and vapor causes severe burns. Harmful if inhaled; relative density 1.19.) in a 500-mL iodine number flask. Chill the solution in a bath for approximately 10 min and, with constant swirling of the flask, add from a 10-mL calibrated buret, 5 more or less 0.01 mL of the bromide-bromate standard solution at the rate of 1 or 2 drops per second. Stopper the flask immediately, shake the contents, place it again in the ice bath, and add 5 mL of Kl solution in the lip of the flask. After 5 min remove the flask from the ice bath and allow the Kl solution to flow into the flask by slowly removing the stopper. Shake vigorously, add 100 mL of water in such a manner as to rinse the stopper, lip and walls of the flask, and titrate promptly with sodium thiosulfate (Na2S2O3) solution. Near the end of the titration, add 1 mL of starch indicator solution and titrate slowly to disappearance of the blue color. Calculate the molarity of the bromide-bromate solution as follows:
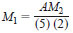
where:
M1 = molarity of the bromide-bromate solution, as Br2,
A = millilitres of Na2S2O3 solution required for titration of the bromide-bromate solution, and,
M2 = molarity of Na2S2O3 solution,
(5) = millilitres of bromide - bromate solution, and
(2) = number of electrons transferred during redox titration of bromide-bromate solution.
Repeat the standardization until duplicate determinations do not differ from the mean by more than more or less 0.002 M.
7.5 Methanol - (Warning - Flammable. Vapor harmful. Can be fatal or cause blindness if swallowed or inhaled. Cannot be made non-poisonous.)
7.6 Potassium Iodide Solution (150 g/L) - Dissolve 150 g of potassium iodide (Kl) in water and dilute to 1 L.
7.7 Sodium Thiosulfate, Standard Solution (0.1 M) - Dissolve 25 g of sodium thiosulfate (Na2S2O3•5H2O) in water and add 0.1 g of sodium carbonate (Na2CO3) to stabilize the solution. Dilute to 1 L and mix thoroughly by shaking.
Standardize by any accepted procedure that determines the molarity with an error not greater than more or less 0.0002. Restandardize at intervals frequent enough to detect changes of 0.0005 in molarity.
7.8 Starch Indication Solution - Mix 5 g of soluble starch with about 3 to 5 mL of water. If desired, add about 0.65 g salicylic acid as preservative. Add the slurry to 500 mL of boiling water and continue boiling for 5 to 10 min. Allow to cool, and decant the clear, supernatant liquid into glass bottles and seal well. Starch solutions (some preserved with salicylic acid) are also commercially available and may be substituted.
7.9 Sulfuric Acid (1 + 5) - Carefully mix one volume of concentrated sulfuric acid (H2SO4, rel dens 1.84) with five volumes of water. (Warning - Poison. Corrosive. Strong oxidizer. Contact with organic material can cause fire. Can be fatal if swallowed.)
7.10 Titration Solvent - Prepare 1 L of titration solvent by mixing the following volumes of materials: 714 mL of glacial acetic acid, 134 mL of 1,1,1-trichloroethane (or dichloromethane), 134 mL of methanol, and 18 mL of H2SO 4(1 + 5).
7.11 1,1,1-Trichloroethane - (Warning - Harmful if inhaled. High concentrations can cause unconsciousness or death. Contact may cause skin irritation and dermatitis.)
7.12 Dichloromethane - (Warning - The replacement of 1,1,1-trichloroethane, an ozone-depleting chemical, is necessary because its manufacture and import has been discontinued. Dichloromethane is temporarily being allowed as an alternative to 1,1,1-trichloroethane until a permanent replacement can be identified and adopted by ASTM. A program to identify and evaluate candidate solvents is currently underway in Subcommittee D02.04.)
NOTE 3 - Commercially available reagents can be used in place of laboratory preparations.

