ASTM D1123 standard test methods for water in engine coolant concentrate by the Karl Fischer reagent method
TEST METHOD A - MANUAL TITRATION
6. Apparatus
6.1 Titration Vessel - For color end point titrations, use a 100 or 250-mL volumetric flask, which need not be calibrated. For instrument end point, a 250-mL flask fitted with interchangeable electrodes (Fig. 1) may be used. This is particularly good for titrations of coolant concentrate that is deeply colored from dye or any other cause. For permanently mounted assemblies, the vessel should have a capacity about equal to that of a 300-mL tall-form beaker and should be provided with a tight-fitting closure to protect the sample and reagent from atmospheric moisture, a stirrer, and a means of adding sample and reagents and removing spent reaction mixture. It is desirable to have a means for cooling the titration vessel to ice temperature.
6.2 Instrument Electrodes, platinum with a surface equivalent to two No. 26 wires, 4.76-mm long. The wires should be 3 to 8 mm apart and so inserted in the vessel that the liquid will cover them.
6.3 Instrument Depolarization Indicator, having an internal resistance of less than 5000 Ω and consisting of a means of impressing and showing a voltage of 20 to 50 mV across the electrodes and capable of indicating a current flow of 10 to 20 µA by means of a galvanometer or radio tuning eye circuit.
6.4 Buret Assembly for Fischer reagent, consisting of a 25 or 50-mL buret connected by means of glass (not rubber) connectors to a source of reagent; several types of automatic dispensing burets may be used. Since the reagent loses strength when exposed to moist air, all vents must be protected against atmospheric moisture by adequate drying tubes containing anhydrous calcium sulfate. All stopcocks and joints should be lubricated with a lubricant not particularly reactive with the reagent.
6.5 Weighing Bottle, of the Lunge or Grethen type, or equivalent.
6.6 Some laboratory equipment suppliers offer a Karl Fischer apparatus. The noted model or its equivalent has been found to be suitable.
7. Reagents
7.1 Purity of Reagents - Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination.
7.2 Unless otherwise indicated, references to water shall be understood to mean reagent water, Type IV, conforming to Specification D1193.
7.3 Karl Fischer Reagent, equivalent to 5 mg of water/mL.
7.4 Methanol (Warning - See 8.1.) - Anhydrous, high purity.
8. Hazards
8.1 Methanol - Poison; flammable; may be fatal or cause blindness if swallowed; cannot be made non-poisonous; harmful if inhaled.
9. Sampling
9.1 A representative sample of the contents of the original container shall be obtained as directed in Test Method D1176; even if two phases are present, the water-insoluble phase should not be separated.
10. Standardization of Reagent
10.1 Standardize the Fischer reagent at least once, prior to use, each day the procedure is used, by either the color or instrument end point (see Section 3) method, using the procedure as used for titrating the sample (Section 11).
10.1.1 Add to each 250 mL flask 25 mL of anhydrous, high purity methanol. Stir rapidly. Titrate with Karl Fischer reagent according to 11.2. Add to the solution 0.15 to 0.18 g of water (to +/- 0.1 mg) by use of an accurately sized syringe. Titrate again and record the volume of titrant used. Repeat standardization two more times.
10.1.2 Calculate the equivalency factor of the reagent in terms of water content per millilitre as follows:
Equivalency factor, F, mg of water/mL = A/B
where:
A = mg of water used in the standardization, and
B = Karl Fischer reagent required, mL.
11. Procedure
11.1 Introduce 30 to 50 mL of the anhydrous high-purity methanol into a 250 mL Erlenmeyer flask, making sure, if an instrument end point apparatus is used, that the electrodes are covered by this amount of methanol. If the color end point is to be determined, make up a second flask as well.
11.2 Adjust the stirrer, if any, to provide adequate mixing without splashing. Titrate the mixture to the instrument end point (3.1.2), or the color end point (3.1.1), with Karl Fischer reagent. If the color end point is to be observed, titrate one flask to match the first. Set aside the first flask as a comparison standard for titrating the sample.
11.3 To the titration mixture thus prepared, add an amount of sample as indicated in Table 1. Exercise care when the sample is transferred so that water is not absorbed from the air, particularly under conditions of high humidity. Again, titrate the mixture with Karl Fischer reagent to the same instrument or color end point previously employed. Record the amount of reagent used to titrate the water in the sample.
NOTE 2 - When using the volumetric flask-type titration vessel in humid climate, place a piece of thin paraffin wax over the mouth of the vessel. Provide a small hole for introducing the buret tip. In less humid climates it is sufficient to lower the tip of the buret deeply into the long neck of the titration flask.
NOTE 3 - In titrating with the volumetric flask-type titration vessel, avoid wetting the stopper and upper end of the flask with either the reagent or the sample solvent. Each time the titration is interrupted, touch the buret tip to the neck of the flask to remove droplets which, if not removed, would absorb moisture from the atmosphere. When the flask is removed from under the buret tip, wipe the tip with a clean dry cloth in a downward motion.
12. Calculation
12.1 Calculate the total water content (free plus apparent) of the sample as follows:
Water, weight % = VF/10M
where:
V = mL of Karl Fischer reagent required by the sample,
F = equivalency factor for Karl Fischer reagent, mg of water per mL of reagent, and
M = sample used, g.
13. Precision and Bias
13.1 Precision - The following data should be used for judging the acceptability of results (95 % probability):
13.1.1 Repeatability - Duplicate results by the same operator should be considered suspect if they differ by more than the following amount:
Repeatability: 0.5 mL of titrant
13.1.2 Reproducibility - The result submitted by one laboratory should not be considered suspect unless it differs from that of another laboratory by more than the following amount:
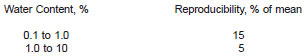
13.2 Bias - Since there is no accepted reference material suitable for determining the bias for the procedure in this test method, bias has not been determined.

