(Equivalent Test Methods: IP 163, ISO 3987, DIN 51575, JIS K 2272, and AFNOR T60-143)
EXPLANATION
Sulfated ash is the residue remaining after the sample has been carbonized, and the residue subsequently treated with sulfuric acid and heated to constant weight.
The sulfated ash may be used to indicate the concentration of known metal-containing additives in new oils. When phosphorus is absent, barium, calcium, magnesium, sodium and potassium are converted to their sulfates and tin (stannic) and zinc to their oxides. Sulfur and chlorine do not interfere, but when phosphorus is present with metals, it remains partially or wholly in the sulfated ash as metal phosphates. Since zinc sulfate slowly decomposes to its oxide at the ignition temperature specified in the method, samples containing zinc may give variable results unless the zinc sulfate is completely converted to the oxide. Magnesium does not react the same as other alkali metals in this test. If magnesium additives are present, the data should be interpreted with caution. Samples containing molybdenum may give low results because molybdenum compounds may not be fully recovered at the temperature of ashing.
Application of this test method to sulfated ash levels below 0.02 % is restricted to oils containing ashless additives. The lower limit of the method is 0.005 % sulfated ash. This test method is not intended for the analysis of used engine oils or oils containing lead. Neither is it recommended for the analysis of nonadditive lubricating oils, for which Test Method D482 should be used. Because of various interelement interferences discussed above, experimentally obtained sulfated ash values may differ from sulfated ash values calculated from elemental analysis. The formation of such nonsulfated species is dependent on the temperature of ashing, time ashed, and the composition of the metal compounds present in the oils. Hence, sulfated ash requirements generally should not be used in product specifications without a clear understanding between the buyer and the seller regarding the unreliability of a sulfated ash value as an indicator of the total metallic compounds.
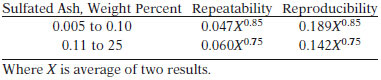
TEST SUMMARY
The sample is ignited and burned until only ash and carbon remain. After cooling, the residue is treated with sulfuric acid and heated at 775° C until oxidation of carbon is complete. The ash is then cooled, re-treated with sulfuric acid, and heated at 775° C to constant weight.